Abstract
Purpose
Layer-specific speckle tissue echocardiography (LS-STE) is a unique technique used to assess coronary microvascular obstruction (CMVO) that may offer more information on the myocardial anatomy of patients with ST-elevation myocardial infarction (STEMI). Cardiovascular magnetic resonance feature tracking (CMR-FT) has also been gaining popularity as a way to evaluate CMVO. The aim of the present study was to directly compare CMVO assessment in STEMI patients using CMR-FT and LS-STE.
Patients and methods
A total of 105 STEMI patients with LS-STE, CMR-FT, and primary percutaneous coronary intervention (PPCI) were included in the study. Longitudinal peak systolic strain (LS), circumferential peak systolic strain (CS), and radial peak systolic strain (RS) were each used to evaluate CMVO using CMR-FT and LS-STE.
Results
Correlation coefficients were 0.56, 0.53, and 0.55 for CMR-FT CS vs. endocardial CS, midcardial CS, and epicardial CS comparisons, respectively, and 0.87, 0.51, and 0.32 for CMR-FT LS vs. endocardial LS, midcardial LS, and epicardial LS comparisons, respectively. Bland-Altman analysis revealed strong inter-modality agreement and little bias in endocardial LS, while the absolute of limited of agreement (LOA) value was 2.28 ± 4.48. The absolutes LOA values were 1.26 ± 11.16, -0.02 ± 12.21, and − 1.3 ± 10.27 for endocardial, midcardial, and epicardial respectively. Intraclass correlation coefficient value of 0.87 showed good reliability in endocardial LS, and moderate reliability with values of 0.71, 0.70, and 0.64 in endocardial, midcardial, and epicardial CS, respectively (all p < 0.001).
Conclusions
CMR-FT is a viable technique for CMVO evaluation in STEMI patients. Endocardial LS showed good reliability for CMR-FT. STEMI patients can undergo LS-STE to assess the CMVO before PPCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The goal of increasing early survival following ST-elevation myocardial infarction (STEMI) has been accomplished in the last few decades [1, 2]. Following the risk stratification procedures is a prerequisite for treatment strategy guidance for diseases like diabetes mellitus and hypertension [3]. Coronary microvascular obstruction (CMVO), intra-myocardial hemorrhage, and intra-myocardial edema also affect the prognosis of the patients with STEMI [4]. The importance of the coronary microvascular system for STEMI prognosis has long been underemphasized. It is one of the major components of the coronary circulation. The efficiency of myocardial revascularization may be hampered by the presence of CMVO with STEMI. In about 30% of patients undergoing primary percutaneous coronary intervention (PPCI) with STEMI, CMVO is to blame for the persistence or recurrence of angina and even heart failure [5]. There are still conflicting results that complete revascularization is better than intensive medical therapy alone in some patients.
It has been shown that CMVO can forecast clinical outcome and post-infarction left ventricular remodeling [6]. Therefore, how to evaluate CMVO has become the problem that needs to be solved immediately. Patients’ clinical presentation and the existence of comorbidities influence the drug treatment methods. An invasive coronary flow reserve (CFR) test is a gold standard used to evaluate functional disorders of coronary microcirculation [7]. It is not widely performed in clinical work due to its complexity, especially in STEMI patients. Coronary microcirculation cannot yet be directly morphologically observed using any imaging modality. However, there are many noteworthy non-invasive tests such as transthoracic Doppler echocardiography, positron emission tomography and cardiovascular magnetic resonance (CMR) [8].
CMR is a non-invasive clinical tool for the assessment of the STEMI patients [9]. Myocardial perfusion can also be evaluated using late gadolinium enhancement (LGE). Its excellent resolution makes it possible to see transmural myocardial flow and is regarded as the gold standard for non-invasively measuring CMVO [10]. Recent research has demonstrated that cardiovascular magnetic resonance feature tracking (CMR-FT) may accurately measure myocardial strain without the requirement for customized pulse sequences or additional scanning time [11].
Speckle tissue echocardiography (STE) is widely used in clinical practice. Myocardial strain, which includes longitudinal peak systolic strain (LS), circumferential peak systolic strain (CS), and radial peak systolic strain (RS), is a quantitative measure of myocardial deformation [12]. Layer-specific STE (LS-STE) analysis has advanced thanks to novel developments in strain imaging techniques. It is utilized for the study of cardiac distortion in clinical practice. LS-STE is a simple, affordable, and painless technique [13]. It may open the door to earlier pathology detection and disease localization in STEMI patients.
The two techniques both have their advantages in detecting the CMVO. Tamarappoo et al. had reported that CMR-FT can predict CMVO as an early sign of LV mechanical dysfunction in STEMI [14]. Garg et al. had demonstrated that STE had the strongest association with CMVO [15]. Strain can also have an impact on how patients with STEMI are categorized in terms of risk. More research is necessary in this area before applying this knowledge in the clinic. CMR-FT is an established technique for predicting CMVO [15]. LS-STE is another method for predicting CMVO due to its versatility, availability, and low cost. In the left ventricle of healthy Chinese participants, Liu et al. discovered that multilayer strain and CMR-FT had a satisfactory connection in circumferential and longitudinal strain in the LV of healthy Chinese participants [16]. Previous studies have reported the prognostic value of CMVO. However, the inter-modality agreement for LS-STE and CMR-FT has not been evaluated, and direct comparison between the novel LS-STE and CMR-FT for CMVO prediction has not been conducted. The present study compares CMR-FT and LS-STE for the assessment of CMVO in STEMI patients.
Materials and methods
Study population and design
This present investigation was conducted retrospectively at a single center. A total of 105 patients who underwent PPCI with STEMI, echocardiography, and CMR procedures between 2020 and 2022 were enrolled in the study. All of the patients received PPCI within 12 h of chest discomfort onset and met the diagnostic requirements for STEMI [17]. The worldwide guidelines suggestions were followed for PPCI. A preliminary TIMI of 0 or 1 anterograde flow in the culprit artery and a subsequent TIMI III flow were shown to each of them. All patients ultimately experienced full revascularization. Patients underwent CMR and echocardiography examinations within 5–7 days following the myocardial infarction.
Patients with a history of myocardial infarction, unsatisfactory segmental imaging, and a lack of echocardiography and CMR examinations were excluded from the study (Fig. 1).
CMR acquisition
All CMR exams were conducted on a 3.0 T magnetic resonance scanner system (Philips Healthcare, Best, The Netherlands) within 5–7 days of successful reperfusion. Cine-CMR tests were carried out while holding breath and were electrocardiogram-triggered. The images were taken in order to evaluate the segmental myocardial function. Then, 8-mm short-axis slices were collected using a prospective electrocardiographically gated gradient-echo sequence with an inversion prepulse after 15 min of intravenous administration of 0.2 mmol/kg gadolinium diethlenetriamine pentacetic acid (Magnevist, Schering, Berlin, Germany).
CMR-FT analysis
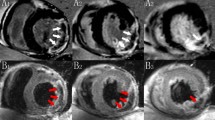
Commercially available Cvi42 software system Segment version 2.0 was used to perform a quantitative evaluation of the CMR-FT images (Calgary, Canada). The endocardium and epicardium of the short-axis section of the layer were manually sketched after determining the locations of the left and right ventricles’ insertion points. The endocardium and epicardium of the remaining short- and long-axis sections were then automatically generated using the AI smart key. The endocardium contours of the portions with plainly mismatched contours were manually sketched after the software automatically recognized the endocardium and epicardium contours of the remaining short-axis segment and long-axis section. Finally, strain analysis parameters were obtained. The values of each strain measure, including the LS, CS, and RS, represent the peak strain values in systole (Fig. 2 and Supplementary Fig. 2).
CMR-LGE acquisition
The collected magnetic resonance delayed enhancement sequence was imported into the cvi42 software. The center of the infarcted myocardium’s low-signal area was classified as a CMVO. The myocardium was divided using a 16-segment model, which was then used as the benchmark to define the myocardial segments for CMVO.
Echocardiography acquisition and LS-STE analysis
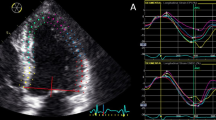
Patients were routinely examined utilizing an S5-1 transducer-equipped commercially available system (Philips IE 33, Philips Medical Systems, Cleveland, OH, USA) while lying in the left lateral decubitus posture. Next, 12-lead electrocardiography and blood pressure readings were taken throughout the evaluation. At the basal, midventricular, and apical levels, two-dimensional grayscale images of the apical four-chamber, apical two-chamber, apical long-axis, and parasternal LV short-axis views were recorded. In order to obtain the images, harmonic (1/3 MHz) B-mode imaging was used at a frame rate of 60–70 frames/s. Three consecutive cardiac cycles were recorded for each view while the patients were holding his or her breath.
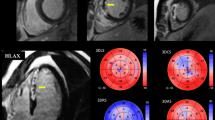
The Q-Lab workstation was used to imported the photos (software 8.1 S Germany). The proper myocardial thickness, the mitral annulus, the LV apex, the LV endocardium, and the epicardium were measured simultaneously. The section boundaries were manually modified if they were unsatisfactory. The software also provided speckle tracking results and automatically separated the heart muscle into the endocardium, mid-myocardium, and epicardium. LS, CS, and RS were calculated for three layers in 16 segments during the three cardiac cycles (Fig. 2 and supplementary Fig. 1).
All of the parameters were included in the RS, endocardial CS (EndoCS), midcardial CS (MidCS), epicardial CS (EpiCS), endocardial LS (EndoLS), midcardial LS (MidLS), and epicardial LS (EpiLS).
Repeatability
Two independent observers used the same echocardiographic and CMR images to determine all of the parameters (inter-observer variability). Similarly, the same observer analyzed all of the images more than three months apart (intra-observer variability).
Statistical analysis
R Studio software version 4.1.3 was used for all statistical analysis, and a p-value of 0.05 was regarded as statistically significant. The mean and standard deviation were used to express continuous data. Bland-Altman plots were used to gauge agreement. Pearson’s correlation coefficient and the intraclass correlation coefficient (ICC) were used to compare the agreements. The mean difference between the CMR-FT and STE datasets was evaluated using the paired sample t-test.
Results
All patients underwent PCI and their coronary angiography results revealed signs of STEMI. The study cohort is represented in Table 1. There were 1680 LV segments in total, however 23 segments were excluded due to low image quality. CMVO was present in 670 infarcted segments in the 105 patients. CMR and echocardiography results were obtained for all patients. There were 670 segments in total (Fig. 1).
Direct comparison of CMR-FT and LS-STE to evaluate CMVO
The Pearson’s correlation, intraclass correlation, and Bland-Altman analyses results between CMR-FT and LS-STE are shown in Table 2. The liner correlation and Bland-Altman analyses plots between CMR-FT and LS-STE are presented in Figs. 3, 4 and 5.
Inter-modality agreement and correlation between CMR-FT and LS-STE derived strain parameters for CS. Notes: A1: Correlation FT-CS vs. EndoCS; B1: Bland Altman: FT-CS vs. EndoCS; A2: Correlation FT-CS vs. MidCS; B2: Bland Altman: FT-CS vs. MidCS; A3: Correlation FT-CS vs. EpiCS; B3: Bland Altman: FT-CS vs. EpiCS
Inter-modality agreement and correlation between CMR-FT and LS-STE derived strain parameters for LS. Notes: A1: Correlation FT-LS vs. EndoLS; B1: Bland Altman: FT-LS vs. EndoLS; A2: Correlation FT-LS vs. MidLS; B2: Bland Altman: FT-LS vs. MidLS; A3: Correlation FT-LS vs. EpiLS; B3: Bland Altman: FT-LS vs. EpiLS
The corresponding values using LS-STE and FTLS were 0.87 for EndoLS vs. FTLS, 0.51 for MidLS vs. FTLS, and 0.32 for EpiLS vs. FTLS comparisons. Compared to FTLS, EndoLS for the CMVO was significantly higher than any other parameters. Agreement between the two techniques was good in a comparison of FTLS vs. EndoLS (bias = 2.28). The reliability was good between FTLS and EndoLS (ICC = 0.87), and moderate between FTLS and MidLS (ICC = 0.56).
The corresponding values for comparisons of FT-CS vs. EndoCS, FT-CS vs. MidCS, FT-CS vs. EpiCS were 0.56, 0.53, 0.55 respectively. The agreement between FT-CS and other STE-CS parameters, such as EndoCS, MidCS, and EpiCS was good (bias = 1.26, -0.02, and − 1.3, respectively). The reliability was moderate for all the three parameters (ICC = 0.71, 0.70, and 0.64, repectively).
The agreement and reliability for FTRS vs. RS was poor due to the lower correlation for RS.
Repeatability
The results for inter- and intra- observer variability are shown in Table 3.
Discussion
The present study’s key conclusion is that FTLS and EndoLS exhibit strong inter-modality agreement in CMVO assessment as measured using Pearson’s correlation coefficient and Bland-Altman analysis. FTLS showed better dependability compared to EndoLS. The agreement and reliability were fair in CS, while RS was inadequate for evaluating CMVO.
Importance of CMVO evaluation
CMVO is linked with a poor prognosis, and varying degrees of CMVO occur in almost all AMI patients receiving PPCI. About 50% of patients experience spontaneous improvement over time [18].
Infarct size, along with ventricular function, clinical events, and cardiovascular mortality, is a key factor in determining a patient’s prognosis. Reversible edema, capillary damage with intramyocardial bleeding, microembolization, and several other symptoms are all present in coronary microcirculation [19]. Coronary microembolization is the AMI patient group’s most likely symptom [20]. However, during PPCI, it is unclear whether protection equipment is necessary during PPCI. In patients with acute myocardial infarction, the use of a mesh-covered embolic protection stent was superior to a standard stent [21]. Above all, numerous particle debris and a number of soluble chemicals are produced during the rupture of atherosclerotic plaque, contributing to poor microvascular perfusion. They all induced CMVO in AMI patients regardless of whether they underwent PPCI. How to measure and protect from the CMVO is an urgent problem. Heusch G et al. found that ischemic preconditioning has been used in patients with pre-infarction angina. However, it can effectively reduce the infarct size and CMVO [22]. Marcos-Garcés V et al. demonstrated that time to reperfusion, GRACE risk score, LV ejection fractions (EF) and CMVO were the novel variables for simple and straightforward major adverse cardiovascular events risk assessment (MACE) after STEMI [23].
The integrity of microcirculation structure and function after myocardial infarction is the basis of myocardial survival. The more abundant the microcirculation, the better the blood supply to the myocardium and the stronger the systolic and diastolic functional reserve. Therefore, we deduced that the strain technique has some diagnostic value in indicating the occurrence of the CMVO in the whole or segmental myocardium. Some studies based on foreign [24, 25] and Chinese [26] populations have also shown that strain analysis has good diagnostic accuracy in distinguishing non-MVO infarcts from MVO infarcts. Our team have already concluded that layer-specific analysis of 2-dimensional speckle tracking echocardiography (LS-2D-STE) can provide additional and reliable diagnostic tools to identify MVO in STEMI patients after reperfusion [27].
Due to their complicated development and high cost, invasive procedures cannot be routinely employed as the gold standard for evaluating CMVO. Safe and precise CMR-FT and LS-STE imaging methods allow for the identification of many CMVO characteristics. In CMVO, STE-derived values provide helpful diagnostic assistance. Additionally, they are significant predictors of functional prognosis in individuals with STEMI and are connected to the presence of CMVO on CMR images. To learn more about the diagnosis, prognosis, and risk stratification of STEMI with CMVO, STE analysis must be taken into account during the ultrasound examination due to its simplicity, speed of execution, low cost, and bedside feasibility [28].
Bland-altman analysis of CMR-FT and LS-STE
Imaging can be easily performed within a routine examination for both LS-STE and CMR-FT. Hence, no extra CMR sequences are required and data analyses can be conveniently conducted using clinically approved software. Routine echocardiography is an economical and easy method to effectively assessCMVO. LS-STE can be more accurate than STE without the extra cost.
According to research by Pankaj Garg et al., CMR-FT is highly correlated with the occurrence of CMVO in STEMI patients. Compared to other CMR metrics, baseline global longitudinal peak systolic strain (GLS) showed a greater correlation with unfavorable LV remodeling [15]. It can reveal important prognostic information after myocardial infarction [29]. When compared to echocardiography, CMR-FT has already been demonstrated to provide more precise measurements of cardiac deformation without the use of a contrast agent. Circumferential peak systolic strain (CS), according to research by Torben Lange et al., is a valuable metric for describing how the distant myocardium responds [30]. According to the previous studies, CMR-FT is always used to evaluate the myocardial function, while strain values indirectly represent the compensatory capacity of the myocardial infarction. CMR-FT is also used to assess the CMVO to allow further risk stratification in patients with STEMI [10].
Good correlation was noted between CMR-FT LS and EndoLS for myocardial strain in the present study. Other LS layers showed moderate and even poor correlation. Consistent with previous studies, LS strain can be an independent predictor for MACE [31]. However, the correlation should be higher in the present study, and the result was not the same. Sun et al. showed that EndoLS was more noticeable than changes in LS strain [32]. First, the subendocardial myocardium had the most extensive damage of the three layers. Second, it could improve the correlation results because all of the segments were explored in previous studies. The present study, only focused on the CMVO segments without considering normal segments. Even though the time of evaluating STEMI patients was decreased, the accuracy of the results increased.
According to Mandoli GE and colleagues, LS-STE can be a novel, useful tool in the echocardiographic assessment of coronary microvascular disease [17]. Previous research has shown that LS-STE results are very good predictors of LV anomalies in individuals with ischemia illness. STE analysis is able to identify aberrant deformations in STEMI patients. Additionally, Mandoli discovered that CS might serve as a new echocardiographic measure for CMVO identification in LS-STE [17].
Good correlation between CMR-FT and STE-derived whole-layer GLS was also demonstrated by Pyrds et al. (n = 50), although with a lower agreement value than that shown in the Bland-Altman analysis results due to a significant bias [33]. CMR-FT generated an LS value that was lower than the EndoLS value. The CMR-FT also monitored the endocardial and epicardial borders, which both reflect the movement of intra-myocardial speckles [34]. This may be a significant reason for the decrease in CMR-FT values. However, the ICC value for EndoLS was significantly higher than that for EpiLS. Reindl, et al. already demonstrated that LS is a good parameter for CMVO detection in STEMI patients with STE and CMR-FT [35]. LS is an independent predictor for CMVO. They demonstrated that the LS is more sensitive to the myocardial disease. EndoLS has a potential application value for a risk stratification tool for STEMI patients with CMVO. Although CMR shows that CMVO is a clear sign of harm, it does not distinguish between myocardial ischemia-reperfusion injury and initial ischemia-related injury. In the present research, EndoLS may be crucial in identifying CMVO in STEMI patients with PPCI.
Moderate correlation was present between CMR-FT CS and multi-layers of CS. Mangion et al., found that CS was superior to other parameters in STE for CMVO detection, even superior to CMR-FT [36]. In the study of correlation between CMR-FT and multilayer strain, the CS with CMR-FT and LS-STE was able to provide important prognostic information for CMVO in STEMI patients. The outcomes were stable because of the disease’s transmural spread to the midmyocardium and epicardium. The outcome was more constant since the CS was more likely to be compromised in STEMI patients than other metrics. According to Leung, et al [37]. CS may be used as a diagnostic tool to evaluate CMVO in STEMI patients. The EF had an impact on the strain, particularly for LS. According to Stathogiannis, et al., [38] areas of scarring dramatically change CS. It is possible that strain from non-contrast CMR can resemble an LGE image with more tuning.
As the study highlight, the results demonstrated that CS and EndoLS can be incorporated to reliably discriminate between CMVO and the infarcted segments. The prognostic value of the multilayer strain was not reported.
Finally, the two methods’ limit of agreements (LOA) values were still wide, which was consistent with previous studies [15]. First of all, there were still a few patients in the studies and the sizeable LOA suggests that inter-modality measurements were not interchangeable [39]. Clinical decisions regarding the use of LS-STE and CMR-FT should be taken into account. Patients with poor echocardiographic image characteristics, such as reverberations, increased field noise, and poor imaging windows, may benefit from CMR-FT use. Superior image quality, broad fields of view, superior volumetric analysis, and improved tissue composition are its main advantages over STE. However, practically is CMR’s key disadvantage compared to echocardiography, which is an affordable and widely used diagnostic method. In addition, LS-STE is convenient and its accuracy is equal to that of CMR-FT. In the clinical work, STEMI patients can undergo LS-STE to assess the CMVO before PPCI, which can help to distinguish between initial ischemia and reperfusion injury.
Limitations
First, the cvi42 measurements for CMR could only be made on the myocardial layers due to software limitations and image acquisition limitations. Second, only one software package was used to complete the strain study. Thus, repeatability between software vendors may be an issue that has to be addressed. Third, all of the patients who underwent CMR and echocardiography examinations were not evaluated on the same day due to clinical restrictions. This might have an impact on EpiLS’ connection. Furthermore, it is important to recognize that an invasive CFR and assessment of microcirculation was not performed in our study. It is also critical to note that AMI patients make up the majority of the study’s participants. Information about healthy volunteers from a larger population is still needed.
Conclusions
The best inter-modality agreement was observed between CMR-FT and LS-STE for the measurement of CMR-FT LS and endocardial LS for all of the parameters. Bland-Altman analysis showed good reliability for EndoLS and moderate for EndoCS, MidCS, EpiCS, and MidLS.
CMVO evaluation in STEMI patients revealed that CMR-FT is a viable technique. EndoLS demonstrated good reliability for CMR-FT during CMVO evaluation using LS-STE. Three parameters in LS-STE CS were moderate compared to those in FTCS. This implies that CS may have a potential value for CMVO prognosis. In the clinical work, STEMI patients can undergo LS-STE to assess the CMVO before PPCI, which can help to distinguish between initial ischemia and reperfusion injury.
Data Availability
The datasets are available by contacting the corresponding author.
Abbreviations
- CFR:
-
Coronary flow reserve
- CMR:
-
Cardiovascular magnetic resonance
- CMR-FT:
-
Cardiovascular magnetic resonance feature tracking
- CMVO:
-
Coronary microvascular obstruction
- CS:
-
Circumferential peak systolic strain
- D to W:
-
Door to wire
- EndoCS:
-
Endocardial CS
- EndoLS:
-
Endocardial LS
- EpiCS:
-
Epicardial CS
- EpiLS:
-
Epicardial LS
- GCS:
-
Peak global circumferential
- GLS:
-
Global longitudinal peak systolic strain
- ICC:
-
Intraclass correlation coefficient
- LAD:
-
Left anterior descending
- LCX:
-
Left circumflex branch
- LGE:
-
Late gadolinium enhancement
- LOA:
-
Limits of agreements
- LS:
-
Longitudinal peak systolic strain
- LS-STE:
-
Layer-specific speckle tissue echocardiography
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- MidCS:
-
Midcardial CS
- MidLS:
-
Midcardial LS
- MACE:
-
Major adverse cardiovascular events risk assessment
- PCI:
-
Percutaneous coronary intervention
- PET:
-
Positron emission tomography
- PPCI:
-
Primary percutaneous coronary intervention
- RCA:
-
Right coronary artery
- ROC:
-
Receiver-operator characteristics
- RS:
-
Radial peak systolic strain
- STE:
-
Speckle tissue echocardiography
- STEMI:
-
ST elevation myocardial infarction
References
Zahler D, Lee-Rozenfeld K, Ravid D, Rozenbaum Z, Banai S, Keren G, Shacham Y (2019) Relation of lowering door-to-balloon time and mortality in ST segment elevation Myocardial Infarction patients undergoing percutaneous coronary intervention. Clin Res Cardiol 108:1053–1058. https://doi.org/10.1007/s00392-019-01438-6
Neumann JT, Goßling A, Sörensen NA, Blankenberg S, Magnussen C, Westermann D (2020) Temporal trends in incidence and outcome of acute coronary syndrome. Clin Res Cardiol 109:1186–1192. https://doi.org/10.1007/s00392-020-01612-1
Reed GW, Rossi JE, Cannon CP (2017) Acute Myocardial Infarction. Lancet 389:197–210. https://doi.org/10.1016/S0140-6736(16)30677-8
Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, Niccoli G, Crea F (2021) Coronary microvascular dysfunction across the Spectrum of Cardiovascular Diseases: JACC State-of-the-art review. J Am Coll Cardiol 78:1352–1371. https://doi.org/10.1016/j.jacc.2021.07.042
Niccoli G, Montone RA, Lanza GA, Crea F (2017) Angina after percutaneous coronary intervention: the need for precision medicine. Int J Cardiol 248:14–19. https://doi.org/10.1016/j.ijcard.2017.07.105
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic Heart Failure: the Task Force for the diagnosis and treatment of acute and chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18:891–975. https://doi.org/10.1002/ejhf.592
Ford TJ, Corcoran D, Berry C (2018) Stable coronary syndromes: pathophysiology, diagnostic advances and therapeutic need. Heart 104:284–292. https://doi.org/10.1136/heartjnl-2017-311446
Rahman H, Corcoran D, Aetesam-Ur-Rahman M, Hoole SP, Berry C, Perera D (2019) Diagnosis of patients with angina and non-obstructive coronary Disease in the catheter laboratory. Heart 105:1536–1542. https://doi.org/10.1136/heartjnl-2019-315042
Feher A, Sinusas AJ (2017) Quantitative Assessment of coronary microvascular function: dynamic single-Photon Emission Computed Tomography, Positron Emission Tomography, Ultrasound, computed Tomography, and magnetic resonance imaging. https://doi.org/10.1161/CIRCIMAGING.117.006427. Circ Cardiovasc Imaging 10
Indorkar R, Kwong RY, Romano S, White BE, Chia RC, Trybula M, Evans K, Shenoy C, Farzaneh-Far A (2019) Global coronary Flow Reserve measured during stress Cardiac magnetic resonance imaging is an Independent predictor of adverse Cardiovascular events. JACC Cardiovasc Imaging 12:1686–1695. https://doi.org/10.1016/j.jcmg.2018.08.018
Mangion K, Carrick D, Clerfond G, Rush C, McComb C, Oldroyd KG, Petrie MC, Eteiba H, Lindsay M, McEntegart M, Hood S, Watkins S, Davie A, Auger DA, Zhong X, Epstein FH, Haig CE, Berry C (2019) Predictors of segmental myocardial functional recovery in patients after an acute ST-Elevation Myocardial Infarction. Eur J Radiol 112:121–129. https://doi.org/10.1016/j.ejrad.2019.01.010
Ananthapadmanabhan S, Vo G, Nguyen T, Dimitri H, Otton J (2021) Direct comparison of multilayer left ventricular global longitudinal strain using CMR feature tracking and speckle tracking echocardiography. BMC Cardiovasc Disord 21:107. https://doi.org/10.1186/s12872-021-01916-8
Claus P, Omar A, Pedrizzetti G, Sengupta PP, Nagel E (2015) Tissue Tracking Technology for assessing Cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging 8:1444–1460. https://doi.org/10.1016/j.jcmg.2015.11.001
Tamarappoo B, Samuel TJ, Elboudwarej O, Thomson L, Aldiwani H, Wei J, Mehta P, Cheng S, Sharif B, AlBadri A, Handberg EM, Petersen J, Pepine CJ, Nelson MD, Bairey Merz CN (2021) Left ventricular circumferential strain and coronary microvascular dysfunction: a report from the women’s ischemia syndrome evaluation coronary vascular dysfunction (WISE-CVD) project. Int J Cardiol 327:25–30. https://doi.org/10.1016/j.ijcard.2020.11.006
Garg P, Kidambi A, Swoboda PP, Foley JR, Musa TA, Ripley DP, Erhayiem B, Dobson LE, McDiarmid AK, Fent GJ, Haaf P, Greenwood JP, Plein S (2017) The role of left ventricular deformation in the assessment of microvascular obstruction and intramyocardial haemorrhage. Int J Cardiovasc Imaging 33:361–370. https://doi.org/10.1007/s10554-016-1006-x
Liu H, Yang D, Wan K, Luo Y, Sun JY, Zhang TJ, Li WH, Greiser A, Jolly MP, Zhang Q, Chen YC (2017) Distribution pattern of left-ventricular myocardial strain analyzed by a cine MRI based deformation registration algorithm in healthy Chinese volunteers. Sci Rep 7:45314. https://doi.org/10.1038/srep45314
Mandoli GE, Cameli M, Minardi S, Crudele F, Lunghetti S, Mondillo S (2018) Layer-specific strain in dipyridamole stress echo: a new tool for the diagnosis of microvascular angina. Echocardiography 35:2005–2013. https://doi.org/10.1111/echo.14180
Borlotti A, Jerosch-Herold M, Liu D, Viliani D, Bracco A, Alkhalil M, De Maria GL, Channon KM, Banning AP, Choudhury RP, Neubauer S, Kharbanda RK, Dall’Armellina E (2019) Acute Microvascular Impairment Post-reperfused STEMI is reversible and has additional clinical predictive value: a CMR OxAMI Study. JACC Cardiovasc Imaging 12:1783–1793. https://doi.org/10.1016/j.jcmg.2018.10.028
Heusch G (2016) The coronary circulation as a target of Cardioprotection. Circ Res 118:1643–1658. https://doi.org/10.1161/CIRCRESAHA.116.308640
Heusch G (2019) Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol 114:45. https://doi.org/10.1007/s00395-019-0756-8
Stone GW, Abizaid A, Silber S, Dizon JM, Merkely B, Costa RA, Kornowski R, Abizaid A, Wojdyła R, Maehara A, Dressler O, Brener SJ, Bar E, Dudek D (2012) Prospective, randomized, multicenter evaluation of a polyethylene terephthalate Micronet Mesh-Covered Stent (MGuard) in ST-Segment Elevation Myocardial Infarction: the MASTER Trial. J Am Coll Cardiol 60:1975–1984. https://doi.org/10.1016/j.jacc.2012.09.004
Heusch G, Rassaf T (2016) Time to give up on Cardioprotection? A critical Appraisal of Clinical studies on ischemic Pre-, Post-, and remote conditioning. Circ Res 119:676–695. https://doi.org/10.1161/CIRCRESAHA.116.308736
Marcos-Garcés V, Perez N, Gavara J, Lopez-Lereu MP, Monmeneu JV, Rios-Navarro C, de Dios E, Merenciano-González H, Gabaldon-Pérez A, Cànoves J, Racugno P, Bonanad C, Minana G, Nunez J, Moratal D, Chorro FJ, Valente F, Lorenzatti D, Ortiz-Pérez JT, Rodríguez-Palomares JF, Bodi V (2022) Risk score for early risk prediction by cardiac magnetic resonance after acute Myocardial Infarction. Int J Cardiol 349:150–154. https://doi.org/10.1016/j.ijcard.2021.11.050
Gräni C, Stark AW, Fischer K, Fürholz M, Wahl A, Erne SA, Huber AT, Guensch DP, Vollenbroich R, Ruberti A, Dobner S, Heg D, Windecker S, Lanz J, Pilgrim T (2022) Diagnostic performance of cardiac magnetic resonance segmental myocardial strain for detecting microvascular obstruction and late gadolinium enhancement in patients presenting after a ST-elevation Myocardial Infarction. Front Cardiovasc Med 9:909204. https://doi.org/10.3389/fcvm.2022.909204
Everaars H, Robbers L, Götte M, Croisille P, Hirsch A, Teunissen P, van de Ven PM, van Royen N, Zijlstra F, Piek JJ, van Rossum AC, Nijveldt R (2018) Strain analysis is superior to wall thickening in discriminating between infarcted myocardium with and without microvascular obstruction. Eur Radiol 28:5171–5181. https://doi.org/10.1007/s00330-018-5493-0
Liu T, Wang C, Yin J, Wang L, Xuan H, Yan Y, Chen J, Bao J, Li D, Xu T (2022) Comparison of Diagnostic Value between STE + LDDSE and CMR-FT for evaluating coronary microvascular obstruction in Post-PCI patients for STEMI. Ther Clin Risk Manag 18:813–823. https://doi.org/10.2147/TCRM.S374866
Lu Z, Liu T, Wang C, Xuan H, Yan Y, Chen J, Lu Y, Li D, Xu T (2023) The evaluation of coronary microvascular obstruction in patients with STEMI by cardiac magnetic resonance T2-STIR image and layer-specific analysis of 2-dimensional speckle tracking echocardiography combined with low-dose dobutamine stress echocardiography. Heart Vessels 38:40–48. https://doi.org/10.1007/s00380-022-02131-x
Sperlongano S, D’Amato A, Tagliamonte E, Russo V, Desiderio A, Ilardi F, Muscogiuri G, Esposito G, Pontone G, Esposito G, D’Andrea A (2022) Acute Myocarditis: prognostic role of speckle tracking echocardiography and comparison with cardiac magnetic resonance features. Heart Vessels 37:121–131. https://doi.org/10.1007/s00380-021-01893-0
Khan JN, Nazir SA, Singh A, Shetye A, Lai FY, Peebles C, Wong J, Greenwood JP, McCann GP (2016) Relationship of myocardial strain and markers of Myocardial Injury to Predict Segmental Recovery after Acute ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Imaging 9. https://doi.org/10.1161/CIRCIMAGING.115.003457
Lange T, Stiermaier T, Backhaus SJ, Boom PC, Kowallick JT, de Waha-Thiele S, Lotz J, Kutty S, Bigalke B, Gutberlet M, Feistritzer HJ, Desch S, Hasenfuß G, Thiele H, Eitel I, Schuster A (2021) Functional and prognostic implications of cardiac magnetic resonance feature tracking-derived remote myocardial strain analyses in patients following acute Myocardial Infarction. Clin Res Cardiol 110:270–280. https://doi.org/10.1007/s00392-020-01747-1
Iwahashi N, Kirigaya J, Gohbara M, Abe T, Horii M, Hanajima Y, Toya N, Takahashi H, Minamimoto Y, Kimura Y, Akiyama E, Okada K, Matsuzawa Y, Maejima N, Hibi K, Kosuge M, Ebina T, Tamura K, Kimura K (2021) Global strain measured by three-Dimensional Speckle Tracking Echocardiography is a useful predictor for 10-Year prognosis after a First ST-Elevation Acute Myocardial Infarction. Circ J 85:1735–1743. https://doi.org/10.1253/circj.CJ-21-0183
Sun JP, Liang Y, Zhang F, Chen X, Yuan W, Xu L, Bahler RC, Yan J (2020) Serial assessment of focal myocardial function after percutaneous coronary intervention for ST-elevation Myocardial Infarction: value of layer-specific speckle tracking echocardiography. Echocardiography 37:1413–1421. https://doi.org/10.1111/echo.14772
Pryds K, Larsen AH, Hansen MS, Grøndal A, Tougaard RS, Hansson NH, Clemmensen TS, Løgstrup BB, Wiggers H, Kim WY, Bøtker HE, Nielsen RR (2019) Myocardial strain assessed by feature tracking cardiac magnetic resonance in patients with a variety of Cardiovascular Diseases - a comparison with echocardiography. Sci Rep 9:11296. https://doi.org/10.1038/s41598-019-47775-4
Vancheri F, Longo G, Vancheri S, Henein M (2020) Coronary microvascular dysfunction. J Clin Med 9. https://doi.org/10.3390/jcm9092880
Reindl M, Tiller C, Holzknecht M, Lechner I, Eisner D, Riepl L, Pamminger M, Henninger B, Mayr A, Schwaiger JP, Klug G, Bauer A, Metzler B, Reinstadler SJ (2021) Global longitudinal strain by feature tracking for optimized prediction of adverse remodeling after ST-elevation Myocardial Infarction. Clin Res Cardiol 110:61–71. https://doi.org/10.1007/s00392-020-01649-2
Mangion K, Loughrey CM, Auger DA, McComb C, Lee MM, Corcoran D, McEntegart M, Davie A, Good R, Lindsay M, Eteiba H, Rocchiccioli P, Watkins S, Hood S, Shaukat A, Haig C, Epstein FH, Berry C (2020) Displacement encoding with stimulated echoes enables the identification of Infarct Transmurality Early Postmyocardial Infarction. J Magn Reson Imaging 52:1722–1731. https://doi.org/10.1002/jmri.27295
Leung SW, Ratajczak TM, Abo-Aly M, Shokri E, Abdel-Latif A, Wenk JF (2021) Regional end-systolic circumferential strain demonstrates compensatory segmental contractile function in patients with ST-segment elevation Myocardial Infarction. J Biomech 129:110794. https://doi.org/10.1016/j.jbiomech.2021.110794
Stathogiannis K, Mor-Avi V, Rashedi N, Lang RM, Patel AR (2020) Regional myocardial strain by cardiac magnetic resonance feature tracking for detection of scar in Ischemic Heart Disease. Magn Reson Imaging 68:190–196. https://doi.org/10.1016/j.mri.2020.02.009
Haghayegh S, Kang HA, Khoshnevis S, Smolensky MH, Diller KR (2020) A comprehensive guideline for bland-Altman and intra class correlation calculations to properly compare two methods of measurement and interpret findings. Physiol Meas 41:055012. https://doi.org/10.1088/1361-6579/ab86d6
Author information
Authors and Affiliations
Contributions
CFW and LLW analyzed the data and wrote the manuscript. JY and HCX collected the clinical data and searched the relevant literature. JHC contributed to the use of statistical software. DYL and JHC analyzed the imaging information. XCH and DTX designed and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The author reports no conflicts of interest in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Wang, L., Yin, J. et al. Direct comparison of coronary microvascular obstruction evaluation using CMR feature tracking and layer-specific speckle tracking echocardiography in STEMI patients. Int J Cardiovasc Imaging 40, 237–247 (2024). https://doi.org/10.1007/s10554-023-02998-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02998-5