Abstract
At the present time, right ventricular function in patients with aortic stenosis is insufficiently taken into account in the decision-making process of aortic valve replacement. The aim of our study was to evaluate significance of right ventricular dysfunction in patients with severe aortic stenosis by modern 3D echocardiographic methods. This is prospective analysis of 68 patients with severe high and low-gradient aortic stenosis. We evaluated function of left and right ventricle on the basis of 3D reconstruction. Enddiastolic, endsystolic volumes, ejection fraction and stroke volumes of both chambers were assessed. There were more patients with right ventricular dysfunction in low-gradient group (RVEF < 45%) than in the high-gradient group (63.6% vs 39%, p = 0.02). Low-gradient patients had worse right ventricular function than high-gradient patients (RVEF 36% vs 46%, p = 0.02). There wasn’t any significant correlation between the right ventricular dysfunction and pulmonary hypertension (r = − 0.25, p = 0.036). There was significant correlation between left and right ejection fraction (r = 0.78, p < 0.0001). Multiple regression analysis revealed that the only predictor of right ventricular function is the left ventricular function. According to our results we can state that right ventricular dysfunction is more common in patients with low-gradient than in high-gradient aortic stenosis and the only predictor of right ventricular dysfunction is left ventricular dysfunction, probably based on ventriculo-ventricular interaction. Pulmonary hypertension in patients with severe AS does not predict right ventricular dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the EURObservational Research Programme Valvular Heart Disease II Survey, aortic stenosis (AS) is the most common valvular defect in Europe. Based on the monitoring of 5219 patients in this programme, 2152 patients (41.2%) suffered from severe AS [1]. AS is common mainly in the elderly after the age of 75 with a prevalence of 12%. Severe AS occurs with a prevalence of 1–3% in people over 65 years of age, with an increasing prevalence with age [2, 3]. Despite advances in knowledge of clinical, genetic and molecular mechanisms of the disease, surgical or interventional aortic valve replacement is the only method of treatment [4]. Currently, the severity of AS is assessed by several criteria. The most common is a classification by haemodynamic parameters [(peak velocity, mean gradient, aortic valve area (AVA)]. The patients with severe low-gradient AS belong to a special group. These patients have a low mean gradient (< 40 mmHg) and aortic valve area, which corresponds to severe AS (AVA < 1 cm2). Current recommendations, which define algorithms in the decision-making process regarding aortic valve replacement, don´t take into account the anatomical and functional consequences of AS on other parts of the heart, excluding systolic function of the left ventricle (LV) [5]. Recently, a new anatomical and functional classification of the patients with severe AS has been proposed [6]. In addition to the LV, it takes into account the function of the right ventricle (RV). For decade, the role of the RV has been downplayed by the attribute of a forgotten chamber. Several research studies have confirmed that RV dysfunction in patients with severe AS is a significant predictor of mortality, and RV function should be systematically assessed [7,8,9]. The RV function in the aforementioned research studies was assessed by various parameters of two-dimensional and one-dimensional echocardiography, which are burdened with errors and inaccuracies when assessing the RV function. To date, there aren´t any studies assessing the RV function in patients with severe AS by means of modern 3D echocardiographic methods, which are characterized by higher accuracy compared to 2D echocardiographic methods. Therefore, the aim of this study was to evaluate the role of RV dysfunction in patients with severe AS by modern 3D echocardiographic methods.
Methods: patients
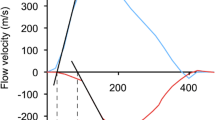
This is prospective analysis of patients with severe AS. Patients enrolled to the study suffered severe AS and had been performed transoesophageal 3D echocardiography (3D TEE) using high quality 3D imaging of the LV and RV (the ultrasound system Siemens Acuson SC 2000 Prime, Mountain View, CA, USA). The patients were examined at the Department of Echocardiography, 1st Department of Cardiology of the East-Slovak Institute for Cardiovascular Diseases and at the Faculty of Medicine, P. J. Safarik University in Košice. Inclusion criteria included severe AS, high-gradient (HG), and low-gradient (LG). High-gradient AS was defined haemodynamically as AS with maximum transvalvular velocity of > 4 m/s, and/or mean gradient of > 40 mmHg and AVA of < 1 cm2. Low-gradient AS was defined haemodynamically as AS with maximum transvalvular velocity of < 4 m/s, mean gradient of < 40 mmHg and AVA of < 1 cm2. Exclusion criteria included pseudostenosis, which was evaluated according to the applicable recommendations, in particular by dobutamine stress echocardiography and also by evaluation of the aortic valve calcium score using CT examination [10]. Besides the primary echocardiographic parameters, which are commonly used in AS evaluation, the LV and RV function on a basis of 3D reconstruction using a prototype program (Siemens, EchoBuildR v3.5.0., Mountain View, CA, USA) for a 3D analysis of the cardiac cavities were assessed. It is an automatic 3D analysis of the LV and RV function where the end-diastolic and end-systolic volumes of the LV and RV, EF and stroke volumes of both ventricles were assessed (Fig. 1). Contours of right ventricule were marked for the 4 chamber, sagital and coronal views in the systole and diastole. Contour drawing used the point-to-point method. After evaluation and confirmation of countours 3D model of RV was obtained. Left ventricule was assessed using a single heartbeat. In left ventricular analysis knowledge-based workflow was used to automatically extract 3 views (apical 4, 3 and 2 chamber) and the transverse view. Pulmonary hypertension (PH) was assessed echocardiographically from tricuspid regurgitation according to applicable recommendations [11].
Prior examination, each patient signed an informed consent form. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statistical methods
Qualitative data were assessed as mean ± standard deviation. Quantitative data were presented in percentage. Data were compared by ANOVA test or by × 2 test. The relationship between particular parameter was assessed by linear or multiple regression analysis. Analyses with value of p < 0.05 were considered as statistically significant.
Results
Primary echocardiographic data are presented in the Table 1.
In the patient cohort, there were significantly more patients with the RV dysfunction (characterized by EF RV < 45%) in the LG AS group (63.6%) than in the HG AS group (39%) (p = 0.02, Fig. 2). There wasn´t any significant correlation between the RV dysfunction and PH (r = − 0.25, p = 0.036, Fig. 3). But the patients with LG AS had worse RV function (36% ± 18) that the patients with HG AS (46% ± 15.9) (p = 0.02, Fig. 4). Multiple regression analysis showed that the LV function is the only predictor of the RV function (p = 0.02, Table 2). Significant correlation was found between LV EF and RV EF (r = 0.78, p < 0.0001, Fig. 5).
Discussion
This study points to the role of RV function assessment using 3D TEE. It is based on the following facts. Nearly half of the patients (47%) with severe AS were diagnosed dysfunctional RV when assessed as RV EF < 45%. Majority of patients with dysfunctional RV was included in the LG AS subgroup (63.6% vs 39%). Similarly, the patients with LG AS had worse RV function than the patients with HG AS (36% ± 18 vs 46% ± 15.9). The LV function (i.e. LV EF) was the only predictor of the RV function. Pulmonary hypertension didn’t show significant impact on the RV function. There was significant correlation between the LV EF and RV EF.
3D echocardiography is currently considered to be an accurate method in the assessment of function and volumes, especially in the case of the LV. Fully automatic assessment modalities of volumes and EF LV were compared with values of volume and EF from magnetic imaging which is considered to be a golden standard [12]. Both, end diastolic and end systolic volumes were moderately undervalued when assessed by 3D echocardiography, but the values of EF were almost identical. Although, LV function assessment by 2D echocardiography is recommended, the clinics, which have experience of using 3D echocardiography, are advised to assess the volumes and LV function by 3D echocardiography. In the case of the RV, according to current recommendations, the RV function is assessed integratively, which implies the use of multiple parameters, such as Doppler, one-dimensional and two-dimensional echocardiography, in the RV function assessment [13]. This results from the inaccuracy of these methodologies. Due to the irregular shape of the RV, the measurement of the volumes and EF RV is more complex than in the case of the LV, which has a simpler geometrical shape. When comparing the 3D echocardiography with MR in volumetry or EF RV assessment, the volumes were significantly undervalued by 3D cardiography, but no significant deviation was recorded when measuring EF by means of 3D echocardiography [14]. According to our knowledge, this is the first study in which LV and RV functions were assessed in relation to AS using 3D echocardiography.
Close relationship between RV and LV function is most likely conditioned by ventricular-ventricular interaction (VVI). The term VVI describes cumulative effect of filling, function, geometry, and synchrony of one ventricle on filling, function, geometry and synchrony of the contralateral ventricle [15]. On usual conditions, a significant part of RV mechanical work is generated by contraction of the LV. VVI plays a significant role mainly in ventricular pressure and volume overload which influences the course and prognosis of the disease. The RV and LV have common interventricular septum (IVS), pericardium and circular muscle fibres which surround both ventricles. The RV free wall is mainly composed of the transverse fibres, which ensure the transversal compression of the RV. It contributes in 20–30% to the RV function. The septum, which belongs to both the RV and LV, is thus biventricular in its nature. It composes approximately 40% of the pure weight of the heart (LV = 38 ± 5%, IVS = 35 ± 4% and RV = 27 ± 1%). The IVS is composed of striated descending and ascending muscle fibres, which form an angle of 60°. Such IVS arrangement/architecture is highly effective because it is involved in the RV function of 70–80%. In patients with pulmonary hypertension but functional IVS, RV function doesn´t decrease significantly, because strong IVS compensates for a dysfunctional RV free wall, which is only partially involved in overall RV function. On the contrary, in patients with both PH and impaired IVS, more significant RV failure occurs, because a larger part of the RV is dysfunctional, i.e. IVS, and thus, the RV free wall can´t compensate IVS dysfunction. Another presumed mechanism of RV and LV interaction is that with significant RV or LV dilatation, the angle between striated muscle fibres decreases (< 60°), and thus effectivity of contraction decreases [16].
The role of the RV function assessment in patients with AS was demonstrated by Eleid et al. [17]. In the cohort of 44 patients who underwent transcatheter aortic valve replacement (TAVR), they found out that immediately after aortic valve replacement and thus relieve of stenosis, the RV function improved in terms of effusion in the RV and systolic velocity of the tricuspid ring. After TAVR, there was no decrease of pressure in the pulmonary trunk, so modification of the RV function wasn´t caused by modification of pulmonary hypertension. The authors assumed that the improvement of RV function was due to VVI. In general, there is a tendency to avoid the intervention when there is a combination of AS and RV dysfunction, although the modification of RV function after TAVR presented in this study suggests the opposite, i.e. the need of intervention; TAVR, which also results to modification of RV function. There are several similar data between our study and the aforementioned study. For example, our cohort consisted of 47% patients with RV dysfunction compared to the study of the aforementioned authors which included 50% of patients with RV dysfunction. Similarly to our findings, the authors didn’t confirm the significant relationship between pulmonary hypertension and RV dysfunction. They claim that the most likely mechanism, which causes RV dysfunction, is LV function during VVI.
Using a solid finite element model of the heart with AS, Wisneski et al. in their study described the highest systolic strain in the area of the basal IVS segment [18]. It can be assumed that in patients with severe AS, the IVS is the most burdened area during pressure overload. This may be the reason for one of the mechanisms of the RV dysfunction.
Forsberg et al. observed the patients with severe AS who underwent TAVR and surgical aortic valve replacement (SAVR) [19]. They assessed the echocardiographic parameters such as atrioventricular plane displacement (AVPD) at the level of the LV lateral wall, IVS, and RV free wall. At these levels, they also monitored the systolic velocities (PVS, peak systolic velocity) assessed by tissue Doppler. These two cohorts of patients were matched by age, sex, and EF LV. AVPD and PSV after TAVR, or SAVR in the area of the LV lateral wall increased in both groups. In the IVS area, AVPD nor PVS increased in the subgroup of patients after SAVR. The RV free wall, AVPD and PSV didn´t change in patients after TAVR but decreased in the cohort of patients after SAVR. The authors conclude that the RV impairment doesn´t occur after TAVR but SAVR. The RV is more protected by TAVR than SAVR. According to some data, the RV dysfunction may persist even 1 year after the cardiosurgical procedure [20]. There are several presumable mechanisms of the RV impairment after the cardiosurgical procedures. Firstly, the drains in front of the RV, post-surgical adhesions, the right ventricle impairment (wall oedema, mechanical trauma) during cannulation for extracorporeal circulation. The LV is more protected by hypothermia than the RV, which is more exposed to the ambient temperature of the surgical theatre, while the procedure is being performed [21]. Secondly, the insufficient perfusion of the RV myocardium during retrograde cardioplegia. Allen et al. assessed perfusion of the RV myocardium using contrastive echocardiography. The study results confirmed that the LV and IVS were threefold to fourfold better perfundated than the RV free wall [22].
Limitations
The main limitation of our study is that pulmonary hypertension wasn´t measured by invasive methods but indirectly from the echocardiographic assumption of pressures in the pulmonary trunk using the tricuspid regurgitation during Doppler examination.
Conclusion
Based on the findings, we can conclude that right ventricle dysfunction is more common in patients with LG AS than in the patients with HG AS. The LV dysfunction is the only predictor of RV dysfunction; most probably it is based on VVI. Pulmonary hypertension in patients with severe AS doesn’t predict worse RV function. Potential clinical application of presented findings is a preference of TAVR over SAVR in the patients with RV dysfunction.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on request.
References
Lung B, Delgado V, Rosenhek R, Price S, Prendergast B, Wendler O et al (2019) Contemporary presentation and management of valvular heart disease: the EURObservational research programme valvular heart disease Ii survey. Circulation 140:1156–1169
Osnabrugge RLJ, Mylotte D, Head SJ, Mieghem NMV, Nkomo VT, LeReun CM et al (2013) Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 62:1002–1012
d’Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson-Stuart J, Birks J et al (2016) Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE population cohort study. Eur Heart J 37:3515–3522
Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thou-Rani VJ et al (2017) 2016 annual report of the society of thoracic surgeons/American college of cardiology transcatheter valve therapy registry. J Am Coll Cardiol 69:1215–1230
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ et al (2017) 2017 ESC/EACTS guidelines for the management of valvular heart disease: the task force for the management of valvular heart disease of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS). Eur Heart J 38:2739–2791
Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA et al (2017) Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J 38:3351–3358
Galli E, Guirette Y, Feneon D, Daudin M, Fournet M, Leguerrier A et al (2015) Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging 16:531–538
Hernandez-Suarez DF, López-Candales A (2017) Subclinical right ventricular dysfunction in patients with severe aortic stenosis: a retrospective case series. Cardiol Ther 6:151–155
Zilberszac R, Gleiss A, Schweitzer R, Bruno P, Andreas M, Stelzmüller M et al (2019) Prognostic value of right ventricular dysfunction and tricuspid regurgitation in patients with severe low-flow low gradient aortic stenosis. SciRep 9:14580
Clavel MA, Magne J, Pibarot P (2015) Low-gradient aortic stenosis. Eur Heart J 37:2645–2657
D’Alto M, Romeo E, Argiento P, D’Andrea A, Vanderpool R, Correra A et al (2017) Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol 168:4058–4062
Spitzer E, Ren B, Zijlstra F, Mieghem NMV, Geleijnse ML (2017) Therole of automated 3D echocardiography for left ventricular ejection fraction assessment. Card Fail Rev 3:97–102
Lang MR, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 16:233–271
Greiner S, Andre F, Heimisch M, Aurich M, Steen H, Katus HA, Mereles D (2019) A closer look at right ventricular 3D volume quantification by transthoracic echocardiography and cardiac MRI. Clin Radiol 74:490.e7-490.e14
Frieberg MF, Redington AN (2018) Rightventricularphysiology, adaptation and failure in congenital and acquired heart disease. Springer International Publishing, Cham
Buckberg G, Hoffman JIE (2014) Right ventricular architecture responsible for mechanical performance: Unifying role of ventricular septum. J Thorac Cardiovasc Surg 148:3166–3171
Eleid MF, Padang R, Pislaru SV, Greason KL, Crestanello J, Nkomo VT et al (2019) Effect of transcatheter aortic valve replacement on right ventricular–pulmonary artery coupling. JACC Cardiovasc Interv 11:2145–2154
Wisneski AD, Wang Y, Deuse T, Hill AC, Pasta S, Sack KL et al (2020) Impact of aortic stenosis on myofiber stress: translational application of left ventricle-aortic coupling simulation. Front Physiol 11:574211
Forsberg LM, Tamas EV, Vanky F, Nielsen NE, Engvall J, Nylander E (2011) Left and right ventricular function in aortic stenosis patients 8 weeks post-transcatheter aortic valve implantation or surgical aortic valve replacement. Eur J Echocardiogr 12:603–611
Alam M, Hedman A, Nordlander R, Samad B (2003) Right ventricular function before and after an uncomplicated coronary artery bypass graft as assessed by pulsed wave Doppler tissue imaging of the tricuspid annulus. Am Heart J 146:520–526
Wranne B, Pinto FJ, Hammarstrom E, St Goar FG, Puryear J, Popp RL (1991) Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J 66:435–442
Allen BS, Winkelmann JW, Hanafy H, Hartz RS, Bolling KS, Ham J et al (1995) Retrograde cardioplegia does not adequately perfuse the right ventricle. J Thorac Cardiovasc Surg 109:1116–1124
Acknowledgements
We want to thank our colleagues at Department of Cardiology for their helpful feedback and support. In particular, we would like to thank Professor Dr Gabriel Valocik for his valuable contributions to our case.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This research received no external funding.
Author information
Authors and Affiliations
Contributions
PF have made a substantial contribution to the concept of the article; MBV and LF revised it critically for important intellectual content; and PZ prepared figures; GV approved the version to be published. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Pavol Fulop, Gabriel Valocik, Marianna Barbierik Vachalcova, Pavol Zenuch and Lenka Filipova declare that they have no conflict of interest.
Ethics approval
Prior examination, each patient signed an informed consent form. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fulop, P., Valocik, G., Barbierik Vachalcova, M. et al. Aortic stenosis and right ventricular dysfunction. Int J Cardiovasc Imaging 40, 299–305 (2024). https://doi.org/10.1007/s10554-023-02986-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02986-9