Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that carries increased risk of cardiovascular disease; however, the underlying pathophysiological mechanisms remain poorly understood. We aimed to investigate the prevalence and degree of myocardial fibrosis in SLE patients and associated disease characteristics. Forty-nine SLE patients (89% female, mean age 52 ± 13 years, median disease duration 19 (11–25) years) and 79 sex-and age-matched healthy controls were included. CMR with T1 mapping was performed on SLE patients and healthy controls. Fifty-one SLE patients received gadolinium contrast for the evaluation of late gadolinium enhancement (LGE) and extra cellular volume (ECV). Multiple linear regression analyses were performed to investigate the association between markers of myocardial fibrosis on CMR (LGE, T1, ECV) and SLE-related variables [clinical disease activity, lupus nephritis, chronic kidney disease, anti-cardiolipin and/or anti-beta-2 glycoprotein I antibodies, and lupus anticoagulant (LAC)] with adjustment for traditional risk factors. T1 values were elevated in SLE patients compared to healthy controls (1031 ± 36 ms vs. 1019 ± 25 ms, p = 0.01). LGE was present in 20% of SLE patients who received gadolinium contrast. On multivariable analysis, LAC was associated with LGE in SLE patients (β = 3.87, p = 0.02). Neither T1 nor ECV associated with SLE disease characteristics; however, there was a trend towards an association between LAC and T1 (β = 16.9, p = 0.08). SLE patients displayed signs of myocardial fibrosis on CMR that were associated with the presence of LAC. These findings support the pathophysiological understanding of LAC as a mediator of microvascular and subsequent myocardial dysfunction.
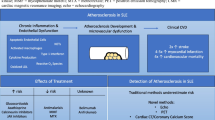
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with systemic lupus erythematosus (SLE) are at increased risk of cardiovascular disease, including myocardial infarction [1, 2], valvular heart disease [3], myocarditis [4], pericarditis [5], and heart failure [6, 7]. Even though SLE patients have increased prevalence of traditional cardiovascular risk factors [8], other SLE-related risk factors are equally believed to contribute such as disease activity, medications, nephritis, antiphospholipid antibodies (aPL), and lupus anticoagulant (LAC) [9]. However, the underlying mechanisms needs further exploration.
Previous studies have reported of an increased prevalence of myocardial fibrosis in SLE [10,11,12,13,14]. Myocardial fibrosis is involved in myocardial remodeling and associated with heart failure [15]. The question therefore seems, if myocardial fibrosis is an underlying cause of cardiac dysfunction observed in SLE patients, including cardiac remodeling, diastolic and systolic dysfunction [16, 17], and which disease factors promote the development.
Two types of myocardial fibrosis exist: replacement fibrosis and diffuse fibrosis. Replacement fibrosis is irreversible and usually develops due to myocyte necrosis (e.g. following ischemia [18]) but is also present in other conditions such as myocarditis [19], non-ischemic dilated cardiomyopathy [20], aortic valve stenosis [21], hypertrophic cardiomyopathy [22], and sarcoidosis [23]. Diffuse fibrosis can precede replacement fibrosis and is regarded as partly reversible. It can be reactive (e.g., due to age and hypertension [24]) or infiltrative (e.g., due to amyloidosis [25]).
Myocardial fibrosis can be estimated by cardiac magnetic resonance (CMR). Replacement fibrosis is visualized by late gadolinium enhancement (LGE) following administration of a gadolinium contrast agent. LGE imaging is part of routine CMR and has been validated against histology in animal models and humans [26,27,28]. The appearance of LGE requires a heterogenous fibrotic distribution, and LGE imaging is therefore unable to detect diffuse myocardial fibrosis. However, T1 and extracellular volume (ECV) mapping are novel semi-automated methods to quantify diffuse myocardial fibrosis. T1 and ECV values correlate with fibrosis on histology [29,30,31], but are also elevated in the presence of edema [32].
We aim to investigate the prevalence of myocardial fibrosis detected by CMR in a population of SLE patients and further associate CMR markers of myocardial fibrosis with biochemical and/or clinical SLE-related risk factors. These include LAC that by our group has been found to be associated with myocardial remodeling and impaired diastolic function [17]. With this, we hope to expand the pathophysiological understanding of cardiac dysfunction in SLE, which seems crucial for better risk stratification and individual follow-up.
Methods
Study population
A total of 79 patients with an established diagnosis of SLE according to the 1997 American College of Rheumatology Revised criteria were included from October 2018 through March 2019 from our tertiary referral center for systemic autoimmune diseases at Copenhagen University Hospital, Rigshospitalet, Denmark. Patients were recruited as previously described [33]. Patients were 18 years of age or older. Pregnancy and ischemic heart disease [i.e., previous primary coronary intervention (PCI) and/or coronary artery bypass grafting (CABG)] were exclusion criteria.
Physical examination, blood samples, and information regarding current medication, previous cardiovascular events, disease activity by the Systemic Lupus erythematosus Disease Activity Index 2000 (SLEDAI-2 K), lupus nephritis (LN), chronic kidney disease stage (CKD), anti-cardiolipin antibodies (aCL), anti-beta2 glycoprotein antibodies (aB2GP), lupus anticoagulant (LAC), organ damage, and traditional cardiovascular risk factors were performed and obtained as also previously described by our group [17].
Further, 79 healthy sex- and age-matched controls were included. The inclusion criterion was age 18–89 years. Exclusion criteria were pregnancy or other contraindications for CMR (e.g., claustrophobia or metallic implants) as well as history of cardiovascular disease (e.g., coronary artery disease, hypertension, and hypercholesterolemia) or other chronic diseases such as chronic obstructive lung disease, chronic kidney disease, and rheumatological disease.
Cardiac magnetic resonance
All patients and healthy controls underwent a CMR examination on a 1.5 T Siemens Magnetom Area Scanner. Steady state free precession images were obtained during breath-hold for the analysis of cardiac morphology and function. Typical cine image parameters were as follows: field-of-view (FOV) 340 mm × 276.4 mm2, in-plane resolution 1.8 × 1.8 × 8 mm3, TR/TE = 42.4/1.11 ms, and flip angle 40°.
Three left ventricular (LV) long-axis planes (two-, three-, and four-chamber views) were acquired as well as a short-axis stack covering both ventricles without gaps.
Late gadolinium enhancement (LGE) imaging was performed only on SLE patients with an estimated eGFR ≥ 45 mL/min/1.73 m2. Gadobutrol (Gadovist, Bayer, Germany) was administered into a peripheral vein at a dose of 0.15 mg/kg. A TI-scout was performed to determine the appropriate TI-time for nulling of the myocardium. LGE images were acquired 12 min following contrast administration and included a full short-axis stack and three long-axis planes of the LV. The TI-time was continuously adjusted during acquisition. In case of LGE, an orthogonal image was acquired to ensure detectability in two planes. Typical LGE image parameters were as follows: FOV 340 × 277.8 m2, in-plane resolution 1.6 × 1.6 × 8 mm3, TR/TE = 700/3.09, and flip angle 25°.
Native T1 mapping was performed using a Look-Locker inversion recovery (MOLLI) sequence following a 5(3)3 protocol. Three short-axis subsets were acquired at the base, mid and apical part of the LV, respectively. T1 mapping images were repeated 10 min post-contrast administration for the generation of ECV mapping images. Typical T1 mapping image parameters were as follows: FOV 360 × 306.7 mm2, in-plane resolution 1.4 × 1.4 × 8 mm3, TR/TE = 280.6/1.12, and flip angle 35°.
Analysis of CMR images was performed using a commercially available software (Circle CVI 42®, Calgary, Canada). LV and right ventricular (RV) volumes were quantified using an AI-function automatically delineating the endo- and epicardium in all slices and phases. A visual validation was subsequently performed, and corrections were performed when necessary. LV and RV end-diastole and end-systole were defined as the phase with the largest and smallest volume, respectively.
Identification and location of LGE was verified by two separate observers in all patients. LGE was characterized in accordance with the Society for Cardiovascular Magnetic Resonance (SCMR) guidelines [34] as either (i) subendocardial or transmural or (ii) mid- and/or epicardial, representing an ischemic or non-ischemic injury, respectively. LGE was quantified by a semi-automated quantification method using either ± 5 SD in cases with an ischemic pattern and ± 3SD in cases with a non-ischemic pattern. LGE was expressed as % volume.
T1 mapping analyses were performed on motion-corrected images, and image quality was evaluated for significant artefacts such as motion and banding artefacts. T1 mapping images with significant artefacts were excluded for further analyses. The endo- and epicardial contours were drawn using an AI-function and corrected if tracings were visually incorrect. A 20% endo- and epicardial offset was chosen to reduce partial-voluming artefacts. A global T1 value was subsequently calculated. ECV maps were computed from pre- and post-contrast T1 images. Hematocrit was measured on the same day as the CMR examination and used in calculations of the ECV using the following formula: \({\text{ECV}}_{{{\text{CMR}}}} ~ = ~\left( {{\text{1}} - {\text{hematocrit}}} \right)~ \times ~\left( {\Delta \left( {{\text{1}}/{\text{T1}}_{{{\text{myo}}}} } \right)/\Delta \left( {{\text{1}}/{\text{T1}}_{{{\text{blood}}}} } \right)} \right)\).
Echocardiography
A standard echocardiographic examination was performed on all patients using a Vivid E95 ultrasound scanner (GE Healthcare Vingmed ultrasound AS, Horten, Norway) and a M5Sc-D Matrix phased array transducer (1.5–4.6 MHz). Performance and analysis of echocardiographic examinations were as previously described [17] and included evaluation of cardiac chamber size, left ventricular ejection fraction (LVEF), and diastolic function.
Statistical analysis
Continuous, normally distributed values are expressed as mean ± standard deviation and non-normally distributed values as median and quartiles. P-values ≤ 0.05 were considered statistically significant. Welch’s t-test was used for comparing normally-distributed data and the Wilcoxon singed-rank test for non-normally distributed data. Correlation between T1 and ECV values was assessed by the Pearson correlation coefficient. Multiple linear and logistic regression analyses were performed to investigate the associations between markers of myocardial fibrosis on CMR (LGE, T1 and ECV) and clinical and biochemical SLE-characteristics (SLEDAI-2 K, LN, CKD, IgM aCL and/or aB2GPI, IgG aCL and/or aB2GPI, and LAC). Further, linear and logistic regressions analyses were performed for the association between markers of myocardial fibrosis on CMR (LGE, T1 and ECV) and biventricular size and function on CMR and echocardiography. All analyses were adjusted for sex, age, hypertension, hypercholesterolemia, and smoking. Non-normally distributed variables were log-transformed to normal distribution before regression analyses. All statistical analyses and Fig. 1 were performed and created using R Statistical Software version 1.2.5001 (R Foundation for Statistical Computing, Vienna, Austria). The graphical abstract and Fig. 2 were created with BioRender.com.
Results
Study population
A total of 70 female and 9 male SLE patients with a mean age of 52 ± 13 years and a disease duration of 19 (11–25) years were included. Demographic and clinical baseline characteristics are presented in Table 1. Further, 79 healthy sex- and age-matched controls were included (89% females, age 49 ± 15 years).
Clinical imaging characterization of SLE patients
Based on CMR, LVEF was reduced (< 50%) in 2 (3%) patients. Diagnosis and grading of diastolic dysfunction by echocardiography were feasible in 70 patients. Nine patients (13%) had diastolic dysfunction of whom 4 patients had mild (grade 1) and 5 patients had moderate (grade 2) diastolic dysfunction.
Comparison of SLE patients with healthy controls
Comparison of CMR parameters in SLE patients and healthy controls are presented in Table 2. LVEF was 2 pp higher in SLE patients (p = 0.05); however, both groups were within normal ranges. RV volumes were 6–8% lower in SLE patients compared to healthy controls; however, due to a similar reduction in end-diastolic and systolic RV volume, there was no difference in RV systolic function between SLE patients and healthy controls.
T1 mapping was feasible in 77 patients. T1 values were 12 ms higher in SLE patients compared to healthy controls (p = 0.01), as illustrated in Fig. 1. With an upper normal reference of 1068 ms, 14% of SLE patients had elevated T1 values.
Late gadolinium enhancement and extracellular volume in SLE patients
Gadolinium contrast was administered to 51 out of 79 SLE patients. Of the 28 patients not receiving gadolinium contrast, 7 patients had a low eGFR (≤ 45 ml/min/1.73 m2), 3 patients refrained from receiving contrast, 4 patients developed claustrophobia or discomfort before administration, and 14 patients did not receive contrast due to logistic reasons (e.g., gadolinium contrast agent not available or no intravenous access). ECV mapping was feasible in 40 patients.
Mean ECV was 28.1 ± 2.8%. LGE was present in 10/51 (20%) SLE patients who received gadolinium contrast. The pattern of LGE was ischemic in 8/10 patients and non-ischemic in 2/10 patients. The median (interquartile range) LGE relative volume was 7 (4–10) %. There was no clear LGE distribution pattern in ischemic patients; however, the two patients with non-ischemic LGE both presented with septal mid-wall LGE. LAC was present in 67% of patients with LGE. Of patients with LGE, 70% were positive for LAC, and of patients without LGE, 32% were positive for LAC (χ2 (1, N = 51) = 7.53, p = 0.01). One of the patients presenting with both LGE and LAC was a 35-year-old male with known antiphospholipid syndrome due to cerebral infarcts and deep vein thrombosis but no history of heart disease. He presented on CMR with extensive subendocardial and transmural LGE in all three coronary territories (Fig. 2), a T1 value of 1102 ms, and an LVEF of 35%. Subsequent CT coronary angiogram was normal, and imaging findings were attributed to multiple micro emboli and/or thrombi.
Late gadolinium enhancement in 35-year-old male patient with systemic lupus erythematosus, antiphospholipid syndrome, and presence of lupus anticoagulant but no history of heart disease. Cardiac magnetic resonance revealed systolic heart failure (left ventricular ejection fraction 35%) and late gadolinium enhancement (white arrows) in all three coronary territories. Coronary angiogram by computed tomography was normal, and the findings were interpreted as resulting from multiple micro emboli and/or thrombi
T1 and ECV trended towards being elevated in patients with LGE; however, not statistically significantly. As expected, T1 and ECV values were strongly correlated (r = 0.6, p < 0.001).
Associations between myocardial fibrosis and ventricular size and function
Increased LV volumes were associated with both increased T1 and ECV values (p < 0.05) (Table 3) when adjusted for age, sex, and traditional risk factors. Further, a lower LVEF was associated with increased T1 values (p < 0.001), and increased left atrial volume index was associated with higher ECV (p = 0.05). LGE was neither associated with LV size nor function besides slightly reduced e’ velocity (p = 0.04). No other significant associations were found between measures of myocardial fibrosis on CMR and biventricular size and function.
Associations between myocardial fibrosis and SLE-specific risk factors
The presence of LAC was associated with the presence of LGE (p = 0.04) in the adjusted regression model (Table 4). Further, there was a trend towards higher T1 in LAC positive patients (p = 0.08), as well as a trend towards a negative association between LN and the presence of LGE (p = 0.0521) and a positive association between higher CKD class and LGE (p = 0.0504). No other associations were found between measures of myocardial fibrosis on CMR and SLE-disease characteristics.
Discussion
In this study controlled for traditional cardiovascular risk factors, we found that SLE patients had (1) signs of myocardial fibrosis estimated with T1 mapping compared to healthy controls, and (2) measures of myocardial fibrosis in SLE patients were associated with the presence of LAC. Given that SLE patients are at increased risk of heart failure not only explained by traditional risk factors, it seems important to identify other underlying SLE-related risk factors.
Prevalence of myocardial fibrosis in SLE patients
Previous studies have reported of LGE in SLE patients with a prevalence ranging from 24 to 61% [10,11,12,13,14, 35, 36]; however, these were all small studies (< 50 SLE patients) with widely different inclusion- and exclusion criteria. Contrary to our study, most have reported of mainly non-ischemic LGE patterns. This discrepancy is likely due to different patient selection and small sample sizes. In one study [12], patients with ischemic LGE were excluded, and patients were mostly younger [11, 12, 14, 35, 36] with shorter disease duration. Further, the analysis of contrast images are reader and image quality dependent, and especially the distinction between non-ischemic mid-wall LGE and artefacts can be challenging if nulling of the myocardium is not perfect. We might have been more conservative in our detection of non-ischemic LGE, which could explain some of the discrepancy with previous studies. Larger studies are however warranted to establish more robust data. Nevertheless, our findings support current evidence that LGE is highly prevalent in SLE patients. Whether LGE is more prevalent in SLE patients compared to healthy controls remains vastly unknown since gadolinium contrast agents are rarely administered to healthy subjects. However, three case-control studies of SLE patients reported of a 0% LGE-prevalence in the control group [12, 36, 37]: Thus substantially lower than the 20% detected in this study cohort.
In the current study, T1 values were elevated in SLE patients compared to healthy controls. Despite a substantial overlap, 14% of SLE patients were above the upper reference value (1068 ms) of the control group. Even though vastly heterogenous, a few studies have similarly found T1 values to be elevated in SLE patients compared to healthy controls [12, 37,38,39]. In a recent paper by Shalmon et al. [37], SLE patients with suspected myocarditis and antimalarial-induced cardiomyopathy had higher T1 levels compared to controls as well as higher prevalence of LGE. Further, in a retrospective case-series [40] of 11 SLE patients (27% with coronary artery disease, 45% with hypertension, 9% with hyperlipidemia, and 36% with end-stage renal disease) with endomyocardial biopsies, 10 patients had non-specific interstitial fibrosis. T1 mapping is generally considered a technique to quantify diffuse myocardial fibrosis. However, T1 values can be increased in replacement fibrosis as well if T1 images cut through these areas. We did not find T1 nor ECV values to be elevated in patients with LGE compared to patients without LGE despite a tendency for LGE-positive patients to span through a greater range of T1- and ECV-values. This might be either due to (1) a power issue, (2) T1 images not cutting through areas of LGE, which were often small, (3) a difference in underlying pathophysiology causing replacement fibrosis in some patients and diffuse fibrosis in other patients, or that (4) increased T1 values could represent diffuse edema/inflammation and not fibrosis. However, T1 values were not associated with disease activity in our analyses.
Association between LAC and myocardial fibrosis in SLE patients
Our group has recently described an association between the presence of LAC and diastolic impairment as well as cardiac remodeling during a 5-year follow-up of the present SLE cohort [17]. We speculated whether these changes in cardiac size and function were due to a LAC-mediated development of myocardial fibrosis. In this study, LAC was associated with the presence of LGE, but not T1 nor ECV values. Most LGE-patterns were ischemic despite most patients having no history of coronary artery disease, and thereby possibly represent subclinical small-vessel disease such as thrombosis in distal branches of coronary arteries. This supports the hypothesis of a potential link between LAC and microvascular dysfunction [41] and/or atherosclerosis [42] due to endothelial activation and recruitment of platelets, neutrophils, monocytes, and the complement system [43]. However, a definitive diagnosis of microvascular dysfunction usually relies on invasive coronary angiography and/or non-invasive techniques such as positron emission tomography or CMR with a rest and stress myocardial perfusion study [44]. As these tests were out of the scope of this study, we cannot draw a conclusive link between our LGE-findings and microvascular dysfunction.
To our knowledge, only Mavrogeni et al. [36] have investigated the association between LAC and myocardial fibrosis by CMR in SLE patients. They found no association between the presence of LAC and LGE: however, this was in a group of 44 patients with antiphospholipid syndrome, either primary or SLE-related, and with a high prevalence of LAC (80%) and not adjusted for traditional cardiovascular risk factors.
The fact that LAC was not associated with T1 nor ECV values could be a power issue but could also suggest other mechanisms to be responsible for development of diffuse fibrosis and/or edema. Numerous studies have reported of elevated T1 and ECV values as well as LGE in other diseases characterized by chronic inflammation such as rheumatoid arthritis [45], systemic sclerosis [46], and type 2 diabetes [47]. It thus seems evident that multiple factors besides LAC are involved.
Association between myocardial fibrosis and ventricular size and function
Given previous findings of impaired diastolic function and increased LV size in SLE patients [16, 17], we investigated whether these parameters were associated with myocardial fibrosis. LV volumes were not significantly increased in SLE patients compared to healthy controls; however, increased volumes as well as reduced LVEF were associated with increased T1 and ECV values in SLE patients. As myocardial fibrosis is highly prevalent in dilated cardiomyopathy and correlates with prognosis [48], it is tempting to draw similar connections in SLE patients.
RV volumes were reduced in SLE patients compared to healthy controls. Few case-control studies have reported of RV-volumes in SLE-patients. A recent case-control study by Pu et al. [49] found no difference in RV-volumes. Our findings on this might be due to healthy controls being more physically active or an actual SLE-related pathophysiological mechanism.
A few measures of diastolic function on echocardiography were associated with LGE and ECV values (e’ velocity and left atrial volume index, respectively), indicating that myocardial fibrosis in SLE patients does somewhat impair the elasticity, compliance, and recoil function of the LV. However, diastolic function is complex, and no single measure can classify diastolic impairment.
Feature-tracking by CMR (FT-CMR), or myocardial strain, quantifies ventricular contractile function and has gained attention in recent years. FT-CMR has the potential to detect subclinical myocardial dysfunction and has shown to have prognostic value [50]. Impaired strain by FT-CMR has previously been correlated with LGE and histology in hypertrophic cardiomyopathy [51] and LGE extend in ST-segment elevation MI [52]. Only a few studies have associated FT-CMR with myocardial fibrosis in SLE. Wu et al. [53] found that elevated ECV in SLE patients was associated with impaired biventricular strain. Puntmann et al. [12] found that T1 was associated with reduced longitudinal strain in SLE patients, but that LGE was not associated with strain. FT-CMR could thus be an indirect measure of myocardial fibrosis in SLE. FT-CMR has the advantage above LGE that it does not require gadolinium contrast, and further the advantage above T1 that no additional images besides standard cine images are required. It could thus serve as an additional marker in patients undergoing CMR, but further studies on this are warranted.
Clinical implications
LV size and LVEF were not impaired in SLE patients compared to healthy controls. Further, only few had clinically significant heart disease. Most findings including increased T1 values and a high prevalence of LGE thus seem subclinical. However, given that LAC was associated with myocardial fibrosis, a known precursor of heart failure, it seems important to further investigate the prognostic implications of the current findings.
Limitations
In this study, we defined myocardial fibrosis as the presence of LGE and/or increased T1 and ECV values. However, these methods are not 100% specific and could all represent varying degrees of inflammation/edema. Also, despite ischemic and non-ischemic pattern recognition in LGE imaging, the underlying pathophysiology or condition causing myocardial fibrosis (e.g., infarct, amyloid deposition, edema) remains uncertain without an endomyocardial biopsy. To overcome some of these limitations, we will in future studies implement T2 mapping as well, which is more specific for edema.
We chose not to administer gadolinium contrast to healthy controls in this study due to ethical considerations. Consequently, we were not able to compare the prevalence of LGE and ECV values between SLE patients and healthy controls, which would have strengthened our results.
FT-CMR was not included in this study; however, we are determined to investigate this in our cohort in future studies.
This was a cross-sectional study, and we have no prognostic information regarding the clinical significance of the association between LAC and myocardial fibrosis. However, we hope to investigate this in future follow-up studies.
Finally, as previously described by our group [17], there might be some degree of collinearity in our regression analyses.
Conclusion
In this cross-sectional case-control study of 79 SLE patients, myocardial fibrosis was more prevalent in SLE patients and associated with the presence of LAC. Findings were mainly subclinical but given the known link between myocardial fibrosis and heart failure, this study raise awareness of LAC as a potential prognostic marker and pathophysiological mediator behind cardiac dysfunction in SLE.
References
Aviña-Zubieta JA, To F, Vostretsova K, De Vera M, Sayre EC, Esdaile JM (2017) Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res 69:849–856. https://doi.org/10.1002/acr.23018
Hermansen M-L, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S (2017) The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a danish nationwide population-based cohort study. Rheumatol Oxf Engl 56:709–715. https://doi.org/10.1093/rheumatology/kew475
Zuily S, Regnault V, Selton-Suty C, Eschwège V, Bruntz J-F, Bode-Dotto E, De Maistre E, Dotto P, Perret-Guillaume C, Lecompte T, Wahl D (2011) Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: meta-analysis of echocardiographic studies. Circulation 124:215–224. https://doi.org/10.1161/CIRCULATIONAHA.111.028522
Apte M, McGwin GJ, Vilá LM, Kaslow RA, Alarcón GS, Reveille JD (2008) Associated factors and impact of myocarditis in patients with SLE from LUMINA, a multiethnic US cohort. Rheumatol Oxf Engl 47:362–367. https://doi.org/10.1093/rheumatology/kem371
Ryu S, Fu W, Petri MA (2017) Associates and predictors of pleurisy or pericarditis in SLE. Lupus Sci Med 4:e000221. https://doi.org/10.1136/lupus-2017-000221
Yafasova A, Fosbøl EL, Schou M, Baslund B, Faurschou M, Docherty KF, Jhund PS, McMurray JJV, Sun G, Kristensen SL, Torp-Pedersen C, Køber L, Butt JH (2021) Long-term cardiovascular outcomes in systemic lupus erythematosus. J Am Coll Cardiol 77:1717–1727. https://doi.org/10.1016/j.jacc.2021.02.029
Kim CH, Al-Kindi SG, Jandali B, Askari AD, Zacharias M, Oliveira GH (2017) Incidence and risk of heart failure in systemic lupus erythematosus. Heart Br Card Soc 103:227–233. https://doi.org/10.1136/heartjnl-2016-309561
Bruce IN, Urowitz MB, Gladman DD, Ibañez D, Steiner G (2003) Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto risk factor study. Arthitis Rheum 48:3159–3167. https://doi.org/10.1002/art.11296
Ballocca F, D’Ascenzo F, Moretti C, Omedè P, Cerrato E, Barbero U, Abbate A, Bertero MT, Zoccai GB, Gaita F (2015) Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Eur J Prev Cardiol 22:1435–1441. https://doi.org/10.1177/2047487314546826
O’Neill SG, Woldman S, Bailliard F, Norman W, McEwan J, Isenberg DA, Taylor AM, Rahman A (2009) Cardiac magnetic resonance imaging in patients with systemic lupus erythematosus. Ann Rheum Dis 68:1478–1481. https://doi.org/10.1136/ard.2008.098053
Abdel-Aty H, Siegle N, Natusch A, Gromnica-Ihle E, Wassmuth R, Dietz R, Schulz-Menger J (2008) Myocardial tissue characterization in systemic lupus erythematosus: value of a comprehensive cardiovascular magnetic resonance approach. Lupus 17:561–567. https://doi.org/10.1177/0961203308089401
Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, Carr-White G, Sangle S, Schaeffter T, Nagel E (2013) Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging 6:295–301. https://doi.org/10.1161/CIRCIMAGING.112.000151
Seneviratne MG, Grieve SM, Figtree GA, Garsia R, Celermajer DS, Adelstein S, Puranik R (2016) Prevalence, distribution and clinical correlates of myocardial fibrosis in systemic lupus erythematosus: a cardiac magnetic resonance study. Lupus 25:573–581. https://doi.org/10.1177/0961203315622275
du Toit R, Herbst PG, Ackerman C, Pecoraro AJ, du Toit RH, Hassan K, Joubert LH, Reuter H, Doubell AF (2020) Myocardial injury in systemic lupus erythematosus according to cardiac magnetic resonance tissue characterization: clinical and echocardiographic features. Lupus 29:1461–1468. https://doi.org/10.1177/0961203320936748
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 35:569–582. https://doi.org/10.1016/s0735-1097(99)00630-0
Chen J, Tang Y, Zhu M, Xu A (2016) Heart involvement in systemic lupus erythematosus: a systemic review and meta-analysis. Clin Rheumatol 35:2437–2448. https://doi.org/10.1007/s10067-016-3373-z
Myhr KA, Zinglersen AH, Hermansen M-LF, Jepsen MM, Iversen KK, Ngo AT, Pecini R, Jacobsen S (2022) Left ventricular size and function in patients with systemic lupus erythematosus associate with lupus anticoagulant: an echocardiographic follow-up study. J Autoimmun 132:102884. https://doi.org/10.1016/j.jaut.2022.102884
Kim HW, Farzaneh-Far A, Kim RJ (2009) Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol 55:1–16. https://doi.org/10.1016/j.jacc.2009.06.059
Imazio M, Angelico G, Andriani M, Lobetti-Bodoni L, Davini O, Giustetto C, Rinaldi M (2018) Prevalence and prognostic impact of septal late gadolinium enhancement in acute myocarditis with or without preserved left ventricular function. Am J Cardiol 122:1955–1958. https://doi.org/10.1016/j.amjcard.2018.08.038
Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ (2006) Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 48:1977–1985. https://doi.org/10.1016/j.jacc.2006.07.049
Balciunaite G, Skorniakov V, Rimkus A, Zaremba T, Palionis D, Valeviciene N, Aidietis A, Serpytis P, Rucinskas K, Sogaard P, Glaveckaite S (2020) Prevalence and prognostic value of late gadolinium enhancement on CMR in aortic stenosis: meta-analysis. Eur Radiol 30:640–651. https://doi.org/10.1007/s00330-019-06386-3
Green JJ, Berger JS, Kramer CM, Salerno M (2012) Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 5:370–377. https://doi.org/10.1016/j.jcmg.2011.11.021
Hulten E, Agarwal V, Cahill M, Cole G, Vita T, Parrish S, Bittencourt MS, Murthy VL, Kwong R, Di Carli MF, Blankstein R (2016) Presence of Late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging 9:e005001. https://doi.org/10.1161/CIRCIMAGING.116.005001
Drazner MH (2011) The progression of hypertensive heart disease. Circulation 123:327–334. https://doi.org/10.1161/CIRCULATIONAHA.108.845792
Pucci A, Aimo A, Musetti V, Barison A, Vergaro G, Genovesi D, Giorgetti A, Masotti S, Arzilli C, Prontera C, Pastormerlo LE, Coceani MA, Ciardetti M, Martini N, Palmieri C, Passino C, Rapezzi C, Emdin M (2021) Amyloid deposits and fibrosis on left ventricular endomyocardial biopsy correlate with extracellular volume in cardiac amyloidosis. J Am Heart Assoc 10:e020358. https://doi.org/10.1161/JAHA.120.020358
Korkusuz H, Esters P, Naguib N, Nour Eldin N-E, Lindemayr S, Huebner F, Koujan A, Bug R, Ackermann H, Vogl TJ (2009) Acute myocarditis in a rat model: late gadolinium enhancement with histopathological correlation. Eur Radiol 19:2672–2678. https://doi.org/10.1007/s00330-009-1454-y
Moon JCC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ (2004) The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 43:2260–2264. https://doi.org/10.1016/j.jacc.2004.03.035
Schelbert EB, Hsu L-Y, Anderson SA, Mohanty BD, Karim SM, Kellman P, Aletras AH, Arai AE (2010) Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging 3:743–752. https://doi.org/10.1161/CIRCIMAGING.108.835793
Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG (2013) Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart Br Card Soc 99:932–937. https://doi.org/10.1136/heartjnl-2012-303052
Zhang L, Yang ZG, Xu H, Yang MX, Xu R, Chen L, Sun R, Miao T, Zhao J, Zhou X, Fu C, Guo Y (2020) Histological validation of cardiovascular magnetic resonance T1 mapping for assessing the evolution of Myocardial Injury in myocardial infarction: an experimental study. Korean J Radiol 21:1294–1304. https://doi.org/10.3348/kjr.2020.0107
de de Meester Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, Pasquet A, Vancraeynest D, Pouleur A-C, Vanoverschelde J-LJ, Gerber BL (2015) Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified look-locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson 17:48. https://doi.org/10.1186/s12968-015-0150-0
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson 14:42. https://doi.org/10.1186/1532-429X-14-42
Zinglersen AH, Iversen KK, Leffers HCB, Laugesen E, Fleischer J, Jacobsen S (2021) Characteristics of cardiovascular autonomic dysfunction and association with quality of life in patients with systemic lupus erythematosus. Lupus Sci Med 8:e000507. https://doi.org/10.1136/lupus-2021-000507
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance: 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): board of trustees task force on standardized post-processing. J Cardiovasc Magn Reson off J Soc Cardiovasc Magn Reson 22:19. https://doi.org/10.1186/s12968-020-00610-6
du Toit R, Reuter H, Walzl G, Snyders C, Chegou NN, Herbst PG, Doubell AF (2021) Serum cytokine levels associated with myocardial injury in systemic lupus erythematosus. Rheumatol Oxf Engl 60:2010–2021. https://doi.org/10.1093/rheumatology/keaa540
Mavrogeni SI, Markousis-Mavrogenis G, Karapanagiotou O, Toutouzas K, Argyriou P, Velitsista S, Kanoupakis G, Apostolou D, Hautemann D, Sfikakis PP, Tektonidou MG (2019) Silent myocardial perfusion abnormalities detected by stress cardiovascular magnetic resonance in antiphospholipid syndrome: a case-control study. J Clin Med 8:1084. https://doi.org/10.3390/jcm8071084
Shalmon T, Thavendiranathan P, Seidman MA, Wald RM, Karur GR, Harvey PJ, Akhtari S, Osuntokun T, Tselios K, Gladman DD, Hanneman K (2023) Cardiac magnetic resonance imaging T1 and T2 mapping in systemic lupus erythematosus in relation to antimalarial treatment. J Thorac Imaging 38:W33–W42. https://doi.org/10.1097/RTI.0000000000000703
Winau L, Hinojar Baydes R, Braner A, Drott U, Burkhardt H, Sangle S, D’Cruz DP, Carr-White G, Marber M, Schnoes K, Arendt C, Klingel K, Vogl TJ, Zeiher AM, Nagel E, Puntmann VO (2018) High-sensitive troponin is associated with subclinical imaging biosignature of inflammatory cardiovascular involvement in systemic lupus erythematosus. Ann Rheum Dis 77:1590–1598. https://doi.org/10.1136/annrheumdis-2018-213661
Wu R, An D-A, Hu J, Jiang M, Guo Q, Xu J-R, Wu L-M (2018) The apparent diffusion coefficient is strongly correlated with extracellular volume, a measure of myocardial fibrosis, and subclinical cardiomyopathy in patients with systemic lupus erythematosus. Acta Radiol Stockh Swed 1987 59:287–295. https://doi.org/10.1177/0284185117717763
Gartshteyn Y, Tamargo M, Fleischer S, Kapoor T, Li J, Askanase A, Winchester R, Geraldino-Pardilla L (2020) Endomyocardial biopsies in the diagnosis of myocardial involvement in systemic lupus erythematosus. Lupus 29:199–204. https://doi.org/10.1177/0961203319897116
Weber BN, Stevens E, Barrett L, Bay C, Sinnette C, Brown JM, Divakaran S, Bibbo C, Hainer J, Dorbala S, Blankstein R, Liao K, Massarotti E, Costenbader K, Di Carli MF (2021) Coronary microvascular dysfunction in systemic lupus erythematosus. J Am Heart Assoc 10:e018555. https://doi.org/10.1161/JAHA.120.018555
Vlachoyiannopoulos PG, Kanellopoulos PG, Ioannidis JPA, Tektonidou MG, Mastorakou I, Moutsopoulos HM (2003) Atherosclerosis in premenopausal women with antiphospholipid syndrome and systemic lupus erythematosus: a controlled study. Rheumatol Oxf Engl 42:645–651. https://doi.org/10.1093/rheumatology/keg182
Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz-Irastorza G, Salmon JE, Shoenfeld Y, Shovman O, Hunt BJ (2018) Antiphospholipid syndrome. Nat Rev Dis Primer 4:17103. https://doi.org/10.1038/nrdp.2017.103
Taqueti VR, Di Carli MF (2018) Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC State-of-the-art review. J Am Coll Cardiol 72:2625–2641. https://doi.org/10.1016/j.jacc.2018.09.042
Ntusi NAB, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, Wordsworth PB, Neubauer S, Karamitsos TD (2015) Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: insights from CMR T1 Mapping., JACC Cardiovasc. Imaging 8:526–536. https://doi.org/10.1016/j.jcmg.2014.12.025
Thuny F, Lovric D, Schnell F, Bergerot C, Ernande L, Cottin V, Derumeaux G, Croisille P (2014) Quantification of myocardial extracellular volume fraction with cardiac MR imaging for early detection of left ventricle involvement in systemic sclerosis. Radiology 271:373–380. https://doi.org/10.1148/radiol.13131280
Liu X, Gao Y, Guo Y-K, Xia C-C, Shi R, Jiang L, Shen M-T, Xie L-J, Peng W-L, Qian W-L, Deng M-Y, Deng L-L, Ren Y, Yang Z-G (2022) Cardiac magnetic resonance T1 mapping for evaluating myocardial fibrosis in patients with type 2 diabetes mellitus: correlation with left ventricular longitudinal diastolic dysfunction. Eur Radiol. https://doi.org/10.1007/s00330-022-08800-9
Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi C, Arzanauskaite M, Lota A, Tayal U, Vassiliou VS, Gregson J, Alpendurada F, Frenneaux MP, Cook SA, Cleland JGF, Pennell DJ, Prasad SK (2019) Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging 12:1645–1655. https://doi.org/10.1016/j.jcmg.2018.07.015
Pu H, Cui B, Liu J, He W, Zhou X, Lin H, Peng L (2022) Characterization and clinical significance of biventricular mechanics in patients with systemic lupus erythematosus by 3T cardiovascular magnetic resonance tissue tracking. Quant Imaging Med Surg 12:1079–1095. https://doi.org/10.21037/qims-21-520
Romano S, Judd RM, Kim RJ, Heitner JF, Shah DJ, Shenoy C, Evans K, Romer B, Salazar P, Farzaneh-Far A (2020) Feature-tracking global longitudinal strain predicts mortality in patients with preserved ejection fraction: a multicenter study. Cardiovasc Imaging 13:940–947. https://doi.org/10.1016/j.jcmg.2019.10.004
Song Y, Bi X, Chen L, Yang K, Chen X, Dong Z, Wang J, Kong X, Zhao K, Wang H, Duru F, Lu M, Ma L, Qiao S, Zhao S (2022) Reduced myocardial septal function assessed by cardiac magnetic resonance feature tracking in patients with hypertrophic obstructive cardiomyopathy: associated with histological myocardial fibrosis and ventricular arrhythmias. Eur Heart J Cardiovasc Imaging 23:1006–1015. https://doi.org/10.1093/ehjci/jeac032
Yu S, Zhou J, Yang K, Chen X, Zheng Y, Zhao K, Song J, Ji K, Zhou P, Yan H, Zhao S (2021) Correlation of myocardial strain and late gadolinium enhancement by cardiac magnetic resonance after a first anterior ST-Segment elevation myocardial infarction. Front Cardiovasc Med 8:705487. https://doi.org/10.3389/fcvm.2021.705487
Wu R, Shi RY, An DAL, Chen BH, Jiang M, Bacyinski A, Han TT, Deen JM, Kaddurah H, Hu J, Xu JR, Wu LM (2020) Biventricular tissue tracking demonstrating associations between left ventricular myocardial extracellular volume, pulmonary artery pressure, and reduced right ventricular ejection fraction in patients with systemic lupus erythematosus using cardiovascular MRI. Clin Radiol 75:237e17-237e25. https://doi.org/10.1016/j.crad.2019.09.136
Funding
Open access funding provided by Royal Library, Copenhagen University Library. This work was supported by Rigshospitalets Research Fund (Rigshospitalets forskningspuljer) and The Danish Rheumatism Association (Gigtforeningen). The funding source were not involved in the study design, data management, writing of the report, or submission for publication.
Author information
Authors and Affiliations
Contributions
KAM: Conceptualization, Formal analysis, Investigation, Writing—Original draft preparation, Visualization. AHZ: Investigation, Project administration, Funding acquisition, Writing—Reviewing and Editing. RP: Writing—Reviewing and Editing, Supervision. SJ: Conceptualization, Methodology, Validation, Formal Analysis, Resources, Writing—Reviewing and Editing, Supervision, Project Administration, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the local ethics committee (H-1-2013-023).
Consent to participate
Written informed consent was obtained from all patients.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Myhr, K.A., Zinglersen, A.H., Pecini, R. et al. Myocardial fibrosis associates with lupus anticoagulant in patients with systemic lupus erythematosus. Int J Cardiovasc Imaging 40, 127–137 (2024). https://doi.org/10.1007/s10554-023-02970-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02970-3