Abstract
Purpose: High triglyceride glucose (TyG) index level is one of the risks for cardiovascular events. The purpose of this research was to examine the correlation of the triglyceride glucose (TyG) index levels with plaque characteristics and calcification types determined by intravascular ultrasound (IVUS) in acute coronary syndrome (ACS) patients. Methods: A total of 234 acute coronary syndromes (ACS) participants who completed intravascular ultrasound (IVUS) and coronary angiography (CAG) were finally enrolled. Results: Logistic regression analysis manifested that the TyG index was independently correlated with the occurrence of coronary calcification, minimum lumen area (MLA) ≤ 4.0 mm², plaque burden (PB) > 70%, and spotty calcification. Taking the lowest group as a reference, the risk of coronary calcification (OR, 2.57; 95%CI, 1.04–6.35; p = 0.040), MLA ≤ 4.0 mm² (OR, 7.32; 95%CI, 2.67–20.01; p < 0.001), PB > 70% (OR, 2.68; 95%CI, 1.04–6.91; p = 0.041), and spotty calcification (OR, 1.48; 95%CI, 0.59–3.71; p = 0.407) was higher in the highest TyG index group. TyG index was converted into a dichotomous variable or a continuous variable for analysis, and we found that a similar result was observed. In addition, optimal predictive models consisting of clinical variables and the TyG index distinctly improved the ability to predict the prevalence of coronary calcification and MLA ≤ 4.0 mm² (p < 0.05). Conclusion: The TyG index may serve as a potential predictor for calcification patterns and plaque vulnerability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance (IR) is a crucial intermediate process of type 2 diabetes mellitus (T2DM) and metabolic disorders, which has been verified to be closely linked to the occurrence and development of coronary atherosclerosis (AS) [1, 2]. In recent years, the TyG index as a new biomarker, calculated from ln [fasting triglycerides (mg/dL) × fasting blood glucose (mg/dL)/2], has been recognized as a practical and reliable alternative biomarker for evaluating IR due to its advantages in the simple calculation method, low cost, timing saving, and popularization [3]. Previous research has demonstrated that a high level of the TyG index is a risk for the occurrence of coronary artery calcification (CAC) and cardiovascular events [4,5,6,7]. However, the effect of the TyG index on calcification types and plaque vulnerability remains unclear. So far, no studies have been designed to investigate this question.
Intravascular ultrasound (IVUS), as an invasive imaging technique performed in the vessels, can accurately identify the shape and type of calcification in coronary artery plaques. According to several IVUS studies, spotty calcification, thin-cap fibroatheroma (TCFA), MLA ≤ 4.0 mm², and PB > 70% are strongly associated with unstable plaques [8,9,10,11,12,13]. The rupture of vulnerable plaques has been widely recognized as the primary cause of ACS and sudden cardiac death [14]. Therefore, timely identification of vulnerable plaques, search for new biomarkers, and intervention treatment is of vital significance for the prevention of ACS, which is also a research hotspot in recent years.
The present research aimed to examine the correlation of the TyG index levels with the plaque characteristics and calcification types in culprit lesions of ACS patients using IVUS.
Methods
Study population
This was a cross-sectional and observational study. Data from the 1st Affiliated Hospital of Dalian Medical University was recorded and statistically analyzed. 338 patients diagnosed with ACS who underwent CAG and IVUS were screened between February 2017 to July 2018. Finally, 104 subjects were excluded and 234 subjects were analyzed (a detailed flow chart was shown in Fig. 1). Furthermore, all subjects were stratified into three groups based on the TyG index levels. Due to the retrospective nature of the study, it was exempt from medical ethics based on the First Affiliated Hospital of Dalian Medical University Ethics Committee.
Clinical and biochemical data
Demographic, medical history, and laboratory data were systematically collected. Medical history included a history of hypertension, diabetes, smoking, stroke, and old myocardial infarction (OMI). All measurements including fasting plasma glucose (FPG), lipid profile, renal function markers, and liver enzyme levels were recorded in the morning after 8 h of fasting. The blood pressure was measured in the brachial artery of the right arm using an automatic sphygmomanometer with appropriate cuff width after a 5-minute rest, and other data were reported by the patient or according to the medical records.
Definitions
Patients were diagnosed with hypertension if they met one of the following conditions: systolic blood pressure ≥ 140mmHg or diastolic blood pressure ≥ 90mmHg at rest, previous diagnosis of hypertension, or use of antihypertensive medications. Diabetes was defined if the patient developed FPG ≥ 7.0mmoL/L within two days, history of diabetes, or used anti-diabetic treatment. Smoking was defined as currently smoking or using cigarettes regularly in the last six months. TyG index was calculated from ln [fasting triglyceride (mg/dL) × FPG (mg/dL)/2].
Coronary angiography image acquisition
Coronary angiography was performed following the latest guidelines. Before surgery, patients were given oral aspirin (at least 100 mg/d). Heparin, which was used throughout the operation, was given to prevent thrombosis. Coronary angiography was performed by experienced operators, and coronary angiography indicators were recorded in detail. The extent of lumen stenosis and severity of coronary artery calcification was assessed on account of previous studies [15]. The segment of the culprit lesions was located by electrocardiogram and coronary angiography results.
Intravascular ultrasound image obtainment
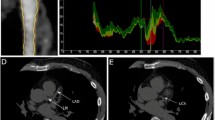
IVUS images were obtained using a commercial IVUS system (iLAB, Boston Scientific Corporation). A 40 MHz, 2.6 F imaging catheter (Atlantis SR Pro or Pro2, Boston Scientific) was advanced 15 mm distal to the culprit lesions and automatically withdrawn at 0.5 mm/s until the aortic ostium was reached. IVUS images were then archived onto CD-ROM or DVD-ROM for offline analysis. All IVUS image analyses were performed using professional offline measurement software (CASS, PIE Medical, Maastricht, the Netherlands). Qualitative and quantitative IVUS grayscale analysis per 1 mm lesion was performed according to the criteria of the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies [16]. IVUS images were interpreted by two experienced IVUS technicians on the CASS workstation, who were unaware of the participant’s clinical data, laboratory data, and angiographic results before processing. Another technician resolved any inconsistencies between the two technicians. The properties of the plaques are as follows: [1] lipid plaques: anechoic or hypoechoic plaques, which were darker than the external elastic membrane (external elastic membrane, EEM) as a reference.; [2] fibrous plaque: moderate echogenic plaque between anechoic lipid plaque and hyperechoic calcified plaque; [3] calcified plaque was defined as an area with a high echoic front compared to the outer membrane, visually highlighted (brighter than the outer membrane), and accompanied by acoustic shadows behind. The extent of calcification was consisting of none, spotty (arc ≤ 90°), mild (91 < arc ≤ 180°), moderate (181 < arc ≤ 270°), and severe (271 < arc ≤ 360°) calcification. Vulnerable plaques were defined as plaques with a lipid angle > 180° and a fibrous cap thickness < 65 μm. Plaque area was defined as the cross-sectional area (CSA) of EEM-lumen CSA. Plaque burden (%) was measured by reference (EEM CSA-luminal CSA) /EEM CSA×100%. MLA was defined as the luminal area at the narrowest point within the vessel where the culprit lesion is located. Calcification length was defined as the distance from the beginning of calcification to the end of calcification on the longitudinal axis of IVUS. Calcification angle was defined as the arc of calcification in degrees per 1 mm of lesion measured through the center of the lumen using an electronic protractor. Based on the conclusions of previous IVUS studies to identify plaque characteristics, the identification indicators of vulnerable plaque in this paper can rely on spotty calcification, MLA ≤ 4.0 mm², and PB > 70% [8, 12, 13]. Several common IVUS images were shown in Fig. 2.
Statistical analysis
The data were expressed as mean ± standard deviation (SD) for normally distributed quantitative data, and the median (interquartile range) for data-skewed variables. Categorical variables were presented as numbers (percentages). To compare the differences among groups stratified by the TyG index, a one-way ANOVA or rank-sum test was used for continuous variables, and the Chi-square test or Fisher’s exact test was used for categorical variables. The correlations of TyG index levels with calcification, MLA ≤ 4.0 mm², PB > 70%, and spotty calcification were analyzed by univariate and multivariate logistic regression analysis while adjusting for potential clinical confounders. The maximum of the Yoden index ([sensitivity + specificity-1]) was used as the cut-off value to calculate the optimum cut-off for calcification, MLA ≤ 4.0 mm², PB > 70%, and spotty calcification. Moreover, the TyG index was divided into binary variables according to the cut-off value and incorporated into the model respectively. The regression results were based on the T1 group with the lowest TyG index as a reference to compare the odds ratio (OR) and 95% confidence interval (CI). In addition, to estimate the predictive ability of the TyG index for coronary calcification, MLA ≤ 4.0 mm², PB > 70%, and spotty calcification in the three different prediction models, the area under the curve (AUC) was calculated through the receiver operating characteristic (ROC) curve analysis. It should be made clear that model 1 included age, sex, history of hypertension, diabetes, current smoking, OMI, TC, and LDL-C, model 2 includes model 1 plus TyG index as a continuous variable, and model 3 includes model 1 plus TyG index as a category variable. All statistical analyses were conducted using SPSS (SPSS version 25.0; IBM, NY). A two-sided p-value < 0.05 was regarded as statistical significance in this study.
Results
Baseline characteristics
The baseline clinical characteristics of the subjects were shown in Table 1. The mean age of the 234 participants (184 men, 78.6%) was 61.2 ± 9.7 years. The incidence of hypertension, diabetes, current smoking, and OMI were 59.0%, 29.9%, 39.3%, and 18.8%, respectively. The levels of uric acid (UA), FPG, total cholesterol (TC), triglyceride (TG), LDL-C, HDL-C, and LPA as well as the prevalence of diabetes and OMI, showed significant differences among the groups. The diagnosis, medication state, and other laboratory data were not markedly different among the three groups.
Angiographic and IVUS imaging analyses
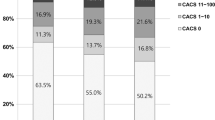
The data of angiographic and IVUS were indicated in Table 2. Significant differences in culprit arteries among the three groups were observed. (p = 0.005). IVUS data revealed that the occurrence of calcified angle ≤ 90°, 91–180°, 181–270°, and 271–360° was 33.3%, 17.9%, 6.0%, and 8.5%, respectively. The incidence of calcified angle ≤ 90° has marked differences among the three groups (p = 0.004). In addition, the incidence of coronary calcification assessed by CAG was 15.4%, but evaluated by IVUS was 65.8%. The incidence of MLA ≤ 4.0 mm² (59.0% vs.67.9% vs.83.3%, p = 0.004), PB > 70% (62.8% vs.79.5% vs.83.3%, p = 0.007), and the level of plaque area (p = 0.006) increased markedly with the increasing of the TyG index.
Association of the TyG index levels with calcification patterns and plaque characteristics
As shown in Table 3, the results from the binary logistic regressions manifested that the TyG index was independently correlated with the occurrence of coronary artery calcification, MLA ≤ 4.0 mm², PB > 70%, and spotty calcification. After adjustments for clinical confounders including age, sex, history of hypertension, diabetes, smoking, OMI, TC levels, and LDL-C levels, these relationships remained significant (p < 0.05) (Model 2). But for spotty calcification, only the results in the second group were statistically significant. Taking the group with the lowest TyG index as a reference, the risk of coronary calcification (OR, 2.57; 95%CI, 1.04–6.35; p = 0.040), MLA ≤ 4.0 mm² (OR, 7.32; 95%CI, 2.67–20.01; p < 0.001), and the PB > 70% (OR, 2.68; 95%CI, 1.04–6.91; p = 0.041) was higher in the group with the highest TyG index. The optimal cut-off values were determined by the receiver operating characteristic (ROC) curve, the TyG index was converted into a dichotomous variable or a continuous variable for analysis, and similar results were obtained.
An optimal predictive model based on the TyG index
ROC results were revealed in Fig. 3. Model 1 included age, sex, history of hypertension, diabetes, current smoking, OMI, TC, and LDL-C. The AUCs of model 1 for calcification was 0.70, spotty calcification was 0.69, MLA ≤ 4.0 mm² was 0.66, and PB > 70% was 0.63. Model 2 (model 1 plus TyG index as a continuous variable) strongly enhanced the predictive ability for the presence of MLA ≤ 4.0 mm², the AUC increased from 0.66 to 0.78 (p = 0.001). Moreover, the AUCs of Model 3 (model 1 plus TyG index as a category variable) had been further increased than Model 1 (calcification: 0.70 to 0.76, p = 0.033; MLA ≤ 4.0 mm²: 0.66 to 0.75, p = 0.023). Nonetheless, these models were not statistically different in predicting the occurrence of spotty calcifications and PB > 70%.
AUCs for the presence of calcification, spotty calcification, MLA ≤ 4.0 mm², and PB > 70%. Model 1: adjusted for age, sex, hypertension, diabetes, current smoking, OMI, TC, and LDL-C. Model 2: model 1 plus TyG index as a continuous variable. Model 3: model 1 plus TyG index as a category variable according to cut-off values evaluated by ROC. The cut-off values of TyG index for calcification, spotty calcification, MLA ≤ 4.0 mm², and PB > 70% were 8.465 mg/dL, 8.565 mg/dL, 8.885 mg/dL, and 8.825 mg/dL, respectively. MLA, minimal lumen area; PB, plaque burden
Discussion
And for all we know, this is the research to examine the correlation of the TyG index levels with plaque characteristics and calcification patterns in ACS patients using IVUS. Our main finding was an obvious correlation of the TyG index with the emergence of coronary calcification, MLA ≤ 4.0 mm², PB > 70%, and spotty calcification, even after adjusting for clinical confounders. Additionally, the risk of coronary calcification, MLA ≤ 4.0 mm², PB > 70% crept up with the TyG index. Another essential result was that the predictive model (TyG index plus clinical risk factors) was superior to other models in predicting coronary artery calcification and plaque instability.
TyG index and calcification patterns
TyG index, identified as an alternative biomarker for insulin resistance (IR), has been reported to be independently related to CAC in healthy adults [7, 17, 18]. Whereas, the correlations of the TyG index with diverse calcification patterns remain uncertain. In this study, we not only verified the correlation of the TyG index with the occurrence of CAC in ACS patients by IVUS but also further found that the TyG index was related to spotty calcification. Despite the underlying mechanism of the correlation between the TyG index and coronary calcification not being elucidated, it is reasonable to speculate that insulin resistance may play a pivotal role, which is consistent with previous reports.
IR was reported to be independently correlated with CAC [19, 20]. The mechanism is that IR leads to vascular damage in vivo by causing vascular REDOX dysfunction, thereby boosting the risk of vascular disease. Vascular damage caused by insulin resistance can be separated into two categories: functional and structural, involving impaired vasodilation mediated by chemical mediators, decreased compliance of the vascular wall (arterial stiffness), the enhanced thickness of the arterial wall, and vascular calcification [21]. Furthermore, IR can promote the formation and progression of atherosclerosis by down-regulating insulin receptor-mediated signaling pathways in vascular intima cells [22, 23].
Previous research has recorded that the TyG index was closely correlated with cardiovascular events or poor prognosis [4, 24]. Nevertheless, the exact mechanism remains uncertain. Fortunately, our research can partially explain this phenomenon. We have verified that the TyG index was independently correlated with spotty calcification in the current study, implying that it has some value in predicting unstable lesions. At the same time, inspired by a pathology-based study, the stability of the lesion depended on the degree and type of calcification. Among them, lesions that appeared densely calcification on the image may be stable plaques (fibrocalcific plaque), while those related to early or unstable plaques were micro, spotty, and sheet-like calcifications [8, 25, 26]. Most importantly, spotty calcifications were more common in ACS than in stable angina [8]. In a word, high levels of the TyG index represent the vulnerable plaques and high-risk individuals to some extent. Unfortunately, in the predictive models incorporating clinical factors and the TyG index, there was no obvious prediction power for the occurrence of spotty calcification. It may be due to the limited sample size of this research, so further research was needed.
TyG index and plaque characteristics
As the main parameters of IVUS, MLA ≤ 4.0 mm², PB > 70%, and TCFAs were used to assess the plaque characteristics, plaque burden, and plaque vulnerability [27]. In the current research, we found that the TyG index was markedly correlated with the occurrence of MLA ≤ 4.0 mm² and PB > 70%. Several related researchers support our results to some extent.
As shown in the formula based on the TyG index, we can infer that the TyG index level is positively correlated with both plasma glucose levels and serum triglyceride (TG) levels. As far as we know, diabetic patients have a higher prevalence of plaque rupture [5], higher lipid burden, larger maximal lipid arc, and a higher incidence of thin-cap fibroatheroma (TCFA). An OCT-based study confirmed an increased incidence of TCFAs and lipid-rich plaques in patients with elevated serum triglyceride levels [28]. Furthermore, an IVUS study demonstrated that coronary plaque volume changes increased with the increase of serum TG levels [29]. In addition, a previous study has revealed that the amount of coronary calcification was positively associated with plaque burden [30]. Meanwhile, the independent correlation between TyG index levels and coronary calcification was obvious in our research. Therefore, the positive relationships between the TyG index and plaque burden were well-founded. Unfortunately, there was not sufficient evidence in this study to discuss the correlation between the TyG index level and TCFA. This may be partly due to the influence of IVUS pixel gray value on resolution, which ultimately leads to the wrong identification of TCFAs.
Limitation
First of all, our study only involved participants from a single center. Given that triglyceride levels varied somewhat by race. Therefore, it was necessary to test whether the TyG index is an appropriate biomarker of CAC and plaque vulnerability in other populations. Second, the subject’s nutritional data was not recorded; therefore, we cannot rule out the potential influence of nutritional habits on blood triglycerides. Third, the TyG index was assessed only once after admission. There were limited data on the dynamics of TyG index levels in the subsequent course of ACS. Fourth, the reason for the high proportion of left main disease was that left main lesions were more suitable for IVUS to guide PCI. Finally, since this is a cross-sectional survey, we were unable to establish a causal relationship between the TyG index and vulnerable plaque. The significance of the TyG index in predicting adverse cardiovascular outcomes also needs to be explored, so further prospective studies are warranted.
Conclusion
A higher TyG index level was markedly correlated with the incidence of spotty calcification, MLA ≤ 4.0 mm², and PB > 70%. According to the results of this study, the TyG index may serve as a non-invasive potential biomarker for the clinical prediction of calcification patterns and plaque features that may represent unstable plaques. This is effective for preventing secondary acute cardiovascular events caused by vulnerable plaques in advance.
Abbreviations
- TyG:
-
Triglyceride glucose
- IR:
-
Insulin resistance
- ACS:
-
Acute coronary syndrome
- CAC:
-
Coronary artery calcification
- IVUS:
-
Intravascular ultrasound
- MLA:
-
Minimum lumen area
- PB:
-
Plaque burden
- TG:
-
Triglyceride
- LDL-C:
-
Low-density lipoprotein cholesterol
References
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA (2018) Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol Aug 31(1):122. https://doi.org/10.1186/s12933-018-0762-4
Beverly JK, Budoff MJ, Atherosclerosis (2020) Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J Diabetes Feb 12(2):102–104. https://doi.org/10.1111/1753-0407.12970
Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W (2020) The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim care diabetes Apr 14(2):161–167. https://doi.org/10.1016/j.pcd.2019.08.004
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA (2016) The TyG index may predict the development of cardiovascular events. Eur J Clin Invest Feb 46(2):189–197. https://doi.org/10.1111/eci.12583
Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH (2019) The triglyceride-glucose index predicts coronary artery Disease Severity and Cardiovascular Outcomes in patients with Non-ST-Segment elevation Acute Coronary Syndrome. Dis Markers 2019:6891537. https://doi.org/10.1155/2019/6891537
Jin JL, Cao YX, Wu LG et al (2018) Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis Nov 10(11):6137–6146. https://doi.org/10.21037/jtd.2018.10.79
Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS (2017) Relationship between the triglyceride glucose index and coronary artery calcification in korean adults. Cardiovasc Diabetol Aug 23(1):108. https://doi.org/10.1186/s12933-017-0589-4
Ehara S, Kobayashi Y, Yoshiyama M et al (2004) Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation Nov 30(22):3424–3429. https://doi.org/10.1161/01.CIR.0000148131.41425.E9
Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S (2013) Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A Jun 25(26):10741–10746. https://doi.org/10.1073/pnas.1308814110
Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW (2017) Coronary artery calcification: from mechanism to Molecular Imaging. JACC Cardiovasc Imaging May 10(5):582–593. https://doi.org/10.1016/j.jcmg.2017.03.005
Shemesh J, Stroh CI, Tenenbaum A et al (1998) Comparison of coronary calcium in stable angina pectoris and in first acute myocardial infarction utilizing double helical computerized tomography. Am J Cardiol Feb 1(3):271–275. https://doi.org/10.1016/s0002-9149(97)00899-0
Vengrenyuk Y, Carlier S, Xanthos S et al (2006) A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci United States Am Oct 3(40):14678–14683. https://doi.org/10.1073/pnas.0606310103
Cardoso L, Kelly-Arnold A, Maldonado N, Laudier D, Weinbaum S (2014) Effect of tissue properties, shape and orientation of microcalcifications on vulnerable cap stability using different hyperelastic constitutive models. J Biomech Mar 3(4):870–877. https://doi.org/10.1016/j.jbiomech.2014.01.010
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol May 20(5):1262–1275. https://doi.org/10.1161/01.atv.20.5.1262
Mintz GS (2015) Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging Apr 8(4):461–471. https://doi.org/10.1016/j.jcmg.2015.02.003
Mintz GS, Nissen SE, Anderson WD et al (Apr 2001) American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus documents. J Am Coll Cardiol 37(5):1478–1492. https://doi.org/10.1016/s0735-1097(01)01175-5
Lee SH, Kwon HS, Park YM et al (2014) Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS ONE 9(2):e90430. https://doi.org/10.1371/journal.pone.0090430
Er LK, Wu S, Chou HH et al (2016) Triglyceride glucose-body Mass Index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE 11(3):e0149731. https://doi.org/10.1371/journal.pone.0149731
Meigs JB, Larson MG, D’Agostino RB et al (2002) Coronary artery calcification in type 2 diabetes and insulin resistance: the framingham offspring study. Diabetes Care Aug 25(8):1313–1319. https://doi.org/10.2337/diacare.25.8.1313
Yamazoe M, Hisamatsu T, Miura K et al (2016) Relationship of insulin resistance to prevalence and progression of coronary artery calcification beyond metabolic Syndrome Components: Shiga Epidemiological Study of subclinical atherosclerosis. Arterioscler Thromb Vasc Biol Aug 36(8):1703–1708. https://doi.org/10.1161/ATVBAHA.116.307612
Adeva-Andany MM, Ameneiros-Rodríguez E, Fernández-Fernández C, Domínguez-Montero A, Funcasta-Calderón R (2019) Insulin resistance is associated with subclinical vascular disease in humans. World J Diabetes Feb 15(2):63–77. https://doi.org/10.4239/wjd.v10.i2.63
Bornfeldt KE, Tabas I (2011) Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab Nov 2(5):575–585. https://doi.org/10.1016/j.cmet.2011.07.015
Rask-Madsen C, Li Q, Freund B et al (2010) Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab May 5(5):379–389. https://doi.org/10.1016/j.cmet.2010.03.013
Wang L, Cong HL, Zhang JX et al (2020) Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol Jun 13(1):80. https://doi.org/10.1186/s12933-020-01054-z
Sakamoto A, Virmani R, Finn AV (2018) Coronary artery calcification: recent developments in our understanding of its pathologic and clinical significance. Curr Opin Cardiol Nov 33(6):645–652. https://doi.org/10.1097/hco.0000000000000558
Jinnouchi H, Sato Y, Sakamoto A et al (2020) Calcium deposition within coronary atherosclerotic lesion: implications for plaque stability. Atherosclerosis Aug 306:85–95. https://doi.org/10.1016/j.atherosclerosis.2020.05.017
Calvert PA, Obaid DR, O’Sullivan M et al (2011) Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable atherosclerosis) study. JACC Cardiovasc Imaging Aug 4(8):894–901. https://doi.org/10.1016/j.jcmg.2011.05.005
Asakura K, Minami Y, Kinoshita D et al (2022) Impact of triglyceride levels on plaque characteristics in patients with coronary artery disease. Int J Cardiol Feb 1 348:134–139. https://doi.org/10.1016/j.ijcard.2021.12.008
Puri R, Nissen SE, Shao M et al (2016) Non-HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol Nov 36(11):2220–2228. https://doi.org/10.1161/ATVBAHA.116.307601
Nicholls SJ, Hsu A, Wolski K et al (2010) Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol May 25(21):2399–2407. https://doi.org/10.1016/j.jacc.2010.02.026
Acknowledgements
The authors would like to thank Yidu Cloud Technology Co., Ltd for the assistance in data searching, extraction, and processing.
Funding
There was no specific grant support for this study.
Author information
Authors and Affiliations
Contributions
Minxian Wang and Xuesong Liu made contributions to the conception, study design, statistical analysis, and drafting of the initial manuscript and served as the equally contributing first authors of the manuscript. Yongkui Ren contributed to material preparation, data acquisition, and analysis. Weili Pan and Da Yin contributed to the conception, study design, review, and revision of the drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was granted an exemption from requiring ethics approval by the First Affiliated Hospital of Dalian Medical University Ethics Committee because this study was a retrospective observational study.
Consent to participate
Informed consent was obtained from all subjects enrolled in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, D., Wang, M., Liu, X. et al. Association of triglyceride glucose index levels with calcification patterns and vulnerability of plaques: an intravascular ultrasound study. Int J Cardiovasc Imaging 39, 2285–2294 (2023). https://doi.org/10.1007/s10554-023-02932-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02932-9