Abstract
We tested the hypothesis that the use of outward displacement of the soft tissue between the apex and the chest wall as seen in TTE, is a sign of apical displacement and would allow for more accurate diagnosis of apical dyskinesis. This is a retrospective study of 123 patients who underwent TTE and cardiac magnetic resonance imaging (MRI) within a time frame of 6 months between 2008 and 2019. 110 subjects were deemed to have good quality studies and included in the final analysis. An observer blinded to the study objectives evaluated the echocardiograms and recorded the presence or absence of apical dyskinesis. Two independent observers evaluated the echocardiograms based on the presence or absence of outward displacement of the overlying tissue at the LV apex. Cardiac MRI was used to validate the presence of apical dyskinesis. The proportion of studies which were identified as having apical dyskinesis with conventional criteria defined as outward movement of the left ventricular apex during systole were compared to those deemed to have dyskinesis based on tissue displacement. By cardiac MRI, 90 patients had apical dyskinesis. Using conventional criteria on TTE interpretation, 21 were diagnosed with apical dyskinesis (23.3%). However, when soft tissue displacement was used as the diagnostic marker of dyskinesis, 78 patients (86.7%) were diagnosed with dyskinesis, p < 0.01. Detection of displacement of soft tissue overlying the LV apex facilitates better recognition of LV apical dyskinesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evaluation of wall motion of left ventricular (LV) segments is an integral component of cardiac imaging. During systole, the myocardium contracts and thickens inwards toward the ventricle cavity. In normal functioning myocardium, the LV motion is synchronous. American Society of Echocardiography (ASE) guidelines recommend visually assessing the LV regional function on transthoracic echocardiography (TTE) using a 17-segment model and grading abnormal wall motion qualitatively using a range of severity from normal to hypokinetic (reduced thickening), akinetic (absent thickening), dyskinetic (systolic thinning or stretching), and aneurysm (apical outpouching) [1]. Dyskinesis is used to describe ventricular muscle segments that bulge outside the LV cavity during systole, as opposed to contracting inwards with the rest of the segments.
The left ventricular apex is usually affected in disease states such as ischemic heart disease with LV apical infarction, hypertrophic cardiomyopathy, and Takotsubo cardiomyopathy. On physical exam, LV apical dyskinesis may be detected as systolic apical displacement during palpation. Although wall thickening of most LV segments can be assessed by transthoracic echocardiography, complete visualization of the LV apex is often difficult and confounded by foreshortening of the LV apex. This is due to nearfield artifact from the proximity of the transducer to the LV apex. These factors may result in underestimation of apical akinesis and dyskinesis by two-dimensional TTE. The outward movement of soft tissue overlying the LV apex seen in patients with apical dyskinesis in the apical 2- and 4-chamber views is associated with infarctions due to left anterior descending (LAD) artery occlusion due to involvement of the blood supply affecting the apex and its proximity to surrounding structures, including the pericardial fat pad, which can transmit dyskinetic segments [2].
Dyskinesis is associated with an infarcted myocardial segment and is more commonly seen at the LV apex. The prevalence and characteristics of apical dyskinesis following myocardial ischemia and necrosis, however, is not well described. Our objective was to test the hypothesis that the use of soft tissue displacement can be identified by TTE (analogous to the findings in physical exam) in the apical views, improving the accuracy of detecting apical dyskinesis in patients suspected to have apical wall motion abnormalities.
Methods
We performed a computerized search on all cardiac MRI and TTE studies performed at Cedars-Sinai Medical Center (CSMC) in Los Angeles, CA, USA, which contained the diagnosis of “apical dyskinesis” in the report. All clinical and imaging data were collected from the electronic medical records. The study met ethical requirements set forth by the Institutional Review Board and approved at Cedars Sinai Medical Center.
Patients who underwent TTE and cardiac MRI within 6 months at CSMC from 2008 to 2019 were included in the study. The time delay between the two studies was < 6 months with average delay being 21 days (range 1–154 days). 103 patients with apical dyskinesis on MRI were initially reviewed; however, 90 were selected as having decent visualization of the LV apex. An additional 20 patients with normal LV ejection fraction, no history of ischemic or nonischemic cardiomyopathy and who underwent cardiac MRI for evaluation of the etiology of premature ventricular beats were selected as controls (Fig. 1).
TTE
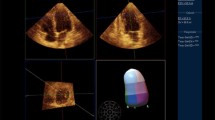
TTE was performed for clinical purposes per standard protocol using standard ultrasound scanners (Philips iE33; Philips Medical Systems; GE Vivid E9, GE Healthcare). Wall motion was assessed with a standard 17-segment model using apical 4-, 3-, and 2- chamber view. Scoring was based on visual assessment of motion of LV segments and apical cap. As the study exams took place before longitudinal strain and contrast echo were used as standard methods to better evaluate the LV apex, it could not be included as part of our evaluation. Apical wall motion was graded by standard echocardiographic analysis on echo reports. The same studies were reviewed by the authors for presence of outward motion of the soft tissue overlying the LV apex (Videos 1, 2). Additional three-dimensional (3D) TTE images were obtained in one example to better illustrate the presence of soft tissue displacement overlying the apex in diastole versus systole (Fig. 1).
Cardiac MRI
CMRI was performed as per standard protocol for a variety of cardiac reasons using a 1.5-Tesla scanner (Avanto, Siemens Healthcare, Erlangen, Germany), with electrocardiogram gating and phased-array surface coil (CP Body Array Flex; Siemens Healthcare), and subjects in the supine position. Steady state free precession images were obtained with axial, vertical long axis and three chamber views. Short axis stack of SSFP cine images was obtained with post-processing LV apical dyskinesis was assessed by a level 3 certified CMRI reader. LV ejection fraction was measured using Circle CVI42 software.
Statistical analysis
Descriptive statistics were summarized as mean or number with percentage. Ejection fraction measurements were further evaluated using mean, median, and interquartile range. Analysis was obtained by Cohen’s kappa statistic using a table comparing initial echocardiographic evidence of dyskinesis to reclassification of dyskinesis on echo read. This statistic is used to define inter-rater reliability for qualitative items. Negative numbers indicate no agreement and the closer the number to 1, the more congruent the observers. Additional evaluation with Fisher’s exact test using a contingency table with p-values were subsequently obtained with significance defined as p < 0.05.
Results
Patient characteristics
Out of the initial 123 participants that were selected as having both TTE and MRI, 110 patients selected for analysis with 90 having MRI-proven dyskinesis and 20 control patients without dyskinesis on MRI (Fig. 2). 84 (76.4%) were men with an average age of 58.3 ± 12.6 years old and a BMI of 26.5 ± 5.2 kg/m2, with an LV ejection fraction of 40.7 ± 16.5% (Table 1). The major indication for echocardiography was either heart failure or coronary artery disease and the major indication for MRI was either myocardial viability or cardiomyopathy.
Flow chart demonstrating patient selection. 123 patients at Cedars-Sinai Medical Center were assessed for inclusion in the study. 13 were excluded for not meeting criteria including well-visualized apex and TTE-MRI interval within 6 months of each other. 90 of the 110 patients had MRI evidence of dyskinesis
LV dyskinesis assessment
Of the 90 patients selected with MRI evidence of apical dyskinesis, 21 (23.3%) had dyskinesis on the initial formal echo read. However, after reclassification with interpretation of soft tissue displacement, 78 (86.7%) were found to have dyskinesis (Fig. 3). This difference was associated with a Cohen’s kappa value of -0.32 indicating no agreement between the initial echo read and reclassification. Additionally, using Fisher exact test, this difference was associated with a p-value of < 0.01 by chi-square analysis (Fig. 4).
Bar graph evaluating dyskinesis results based on standard criteria and with new soft tissue displacement criteria. Y-axis demonstrates number of patients meeting criteria. 69/90 (76.67%) without dyskinesis. 21/90 (23.33%) with dyskinesis on initial interpretation. 12/90 (13.33%) without dyskinesis. 78/90 (86.67%) with dyskinesis with reclassification criteria. K = -0.32, p-value < 0.01
In the control arm with MRI indicating no LV dyskinesis, 4 of the 20 subjects had displacement of the LV apex on TTE.
Discussion
In this retrospective study, we report that patients with apical dyskinesis were more readily identified by using the displacement of the soft tissue overlying the LV apex. This is the first study evaluating this novel diagnostic measure for detecting LV apical dyskinesis.
Apical dyskinesis is commonly observed in ischemic cardiomyopathy and is conventionally diagnosed by visual assessment of LV apical wall motion. In addition to ischemic heart disease, apical wall motion abnormalities can occur in Takotsubo cardiomyopathy, apical hypertrophy, RV apical pacing, vasospastic angina, and nonischemic dilated cardiomyopathy [3,4,5]. Wall motion assessment of the LV apex can be fraught with inaccuracies due to incomplete visualization of the true apex and near field artifacts. While incorporating contrast in wall motion analysis has been reported to improve interobserver agreement of segment visualization in global LV function, abnormalities of apical wall thickening remain underestimated, and their clinical significance may be underappreciated [6]. Cardiac MRI is used as the gold standard modality for assessment of LV wall thickening and volumetric assessment of LV and right ventricular function due to its superior endocardial definition and comparable spatial and temporal resolution when compared to TTE. MRI also allows optimal visualization of the LV apex [7]. We found that 87% of patients were correctly reclassified as having LV dyskinesis on TTE using the criteria of LV apical soft tissue displacement when compared to the initial TTE interpretation. Translational apical wall motion has been used as a measure of dyssynchrony in patients who are being evaluated for cardiac resynchronization therapy [8].
In ischemic cardiomyopathy, LV apical wall motion abnormalities may have prognostic importance as regional LV dyskinesis is associated with adverse cardiovascular outcomes in the first 30 days after a myocardial infarction (MI) [9]. Additionally LV thrombi are more likely to occur in dyskinetic or aneurysmal segments and seem to disappear less often than in akinetic areas [10].
In the control arm with MRI indicating no LV dyskinesis, 4 of the 20 subjects had displacement of the LV apex on TTE. Of note, 3 of those had moderate aortic regurgitation and ascending aortic root dilation, which may have contributed to finding of soft tissue displacement. The remaining patient had evidence of apical-variant hypertrophic cardiomyopathy.
Limitations
This is a single-center retrospective study of patients who underwent CMRI and TTE for clinical indications, which were different for each patient. Furthermore, the TTE and CMRI were not performed specifically for identifying apical dyskinesis which could affect windows and views. As this is an observational study the authors do not know the exact mechanism behind why the apical dyskinesis is present and further studies using our findings should be undertaken to better understand the significance.
As noted above, there was evidence of soft tissue displacement in the echocardiograms of 4 patients without dyskinesis on MRI. It is not clear to the authors why this may be the case but it is important to note that while this novel finding may increase the sensitivity of determining dyskinesis, it may negatively affect specificity.
Conclusion
In this study we report a novel transthoracic echocardiographic finding (displacement of LV apical soft tissue) that facilitates the detection of left ventricular apical dyskinesis.
Abbreviations
- LV:
-
Left ventricle
- TTE:
-
Transthoracic echocardiography
- MRI:
-
Magnetic resonance imaging
- BMI:
-
Body mass index
- EF:
-
Ejection fraction
- ASE:
-
American Society of Echocardiography
- LAD:
-
Left anterior descending
- MI:
-
Myocardial infarction
- CSMC:
-
Cedars-Sinai Medical Center
References
Mitchell C et al (2019) Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 32(1):1–64. https://doi.org/10.1016/j.echo.2018.06.004.
Buckberg GD (1998) Defining the relationship between akinesia and dyskinesia and the cause of left ventricular failure after anterior infarction and reversal of remodeling to restoration. J Thorac Cardiovasc Surg 116(1):47–49. https://doi.org/10.1016/s0022-5223(98)70241-7
Hughes RK et al (2020) Apical hypertrophic cardiomyopathy: the variant less known. J Am Heart Assoc 9(5):e015294. https://doi.org/10.1161/JAHA.119.015294.
Jacob S, Kondur A, Khetarpal V, Afonso L (2009) Reversal of right ventricular apex pacing-induced left ventricular apical dyskinesis: utility of intraoperative 3D echocardiography in resynchronization therapy. Circ Arrhythm Electrophysiol 2(4):e21–e23. https://doi.org/10.1161/CIRCEP.109.867366
Scantlebury DC, Prasad A (2014) Diagnosis of Takotsubo cardiomyopathy. Circ J 78(9):2129–2139. https://doi.org/10.1253/circj.cj-14-0859
Galema TW et al (2011) Contrast echocardiography improves interobserver agreement for wall motion score index and correlation with ejection fraction. Echocardiography 28(5):575–581. https://doi.org/10.1111/j.1540-8175.2010.01379.x
Bellenger NG et al (2000) Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J 21(16):1387–1396. https://doi.org/10.1053/euhj.2000.2011
Szulik M et al (2010) Assessment of apical rocking: a new, integrative approach for selection of candidates for cardiac resynchronization therapy. Eur J Echocardiogr 11(10):863–869. https://doi.org/10.1093/ejechocard/jeq081
Kjoller E, Kober L, Jorgensen S, Torp-Pedersen C (2002) Short and long term prognostic importance of regional dyskinesia versus akinesia in acute myocardial infarction. BMJ Heart 87(5):410–414
Delewi R, Zijlstra F, Piek JJ (2012) Left ventricular thrombus formation after acute myocardial infarction. Heart 98(23):1743–1749. https://doi.org/10.1136/heartjnl-2012-301962
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is an observational study. The Cedars-Sinai Research Ethics Committee has confirmed that no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Video 1: Video demonstrating soft tissue displacement of a 2-chamber view transthoracic echocardiogram (TTE). Supplementary file1 (MP4 632 kb)
Video 2: Video demonstrating soft tissue displacement of a 4-chamber view transthoracic echocardiogram (TTE). Supplementary file2 (MP4 746 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwartz, B.H., Tamarappoo, B.K., Shmueli, H. et al. Soft tissue displacement for detection of left ventricle apical dyskinesis with transthoracic echocardiography. Int J Cardiovasc Imaging 39, 1425–1430 (2023). https://doi.org/10.1007/s10554-023-02856-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02856-4