Abstract
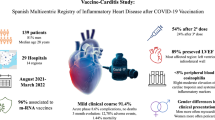
To evaluate clinical and cardiac magnetic resonance (CMR) short-term follow-up (FU) in patients with vaccine-associated myocarditis, pericarditis or myo-pericarditis (VAMP) following COVID-19 vaccination. We retrospectively analyzed 44 patients (2 women, mean age: 31.7 ± 15.1 years) with clinical and CMR manifestations of VAMP, recruited from 13 large tertiary national centers. Inclusion criteria were troponin raise, interval between the last vaccination dose and onset of symptoms < 25 days and symptoms-to-CMR < 20 days. 29/44 patients underwent a short-term FU-CMR with a median time of 3.3 months. Ventricular volumes and CMR findings of cardiac injury were collected in all exams. Mean interval between the last vaccination dose and the onset of symptoms was 6.2 ± 5.6 days. 30/44 patients received a vaccination with Comirnaty, 12/44 with Spikevax, 1/44 with Vaxzevria and 1/44 with Janssen (18 after the first dose of vaccine, 20 after the second and 6 after the “booster” dose). Chest pain was the most frequent symptom (41/44), followed by fever (29/44), myalgia (17/44), dyspnea (13/44) and palpitations (11/44). At baseline, left ventricular ejection fraction (LV-EF) was reduced in 7 patients; wall motion abnormalities have been detected in 10. Myocardial edema was found in 35 (79.5%) and LGE in 40 (90.9%) patients. Clinical FU revealed symptoms persistence in 8/44 patients. At FU-CMR, LV-EF was reduced only in 2 patients, myocardial edema was present in 8/29 patients and LGE in 26/29. VAMPs appear to have a mild clinical presentation, with self-limiting course and resolution of CMR signs of active inflammation at short-term follow-up in most of the cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since late 2020, various types of vaccines with different vectors and mechanisms of action have been authorized and administered for immunization against COVID-19 and in about one and a half years, approximately 5.28 billion people received at least one dose of vaccine [1].
These vaccines demonstrated a strong safety profile, with extremely low rate of side effects and complications. Those include rare cases of vaccine-associated myocarditis or pericarditis (VAMP), mainly affecting young male individuals with higher risk within the first weeks from the second dose and using mRNA-based vaccines (source: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html).
The real frequency and pathogenetic mechanisms underlying the myocardial and pericardial inflammation following vaccination are still poorly understood and highly debated [2,3,4].
In a recent meta-analysis, pooled incidence of myocarditis following COVID-19 vaccine on an overall cohort of 17,704,413 subjects was 35/million over an observation period of about two years, with only a single fatal case [4]. Although not uncommon, VAMPs are less frequent than COVID-19 related myocarditis [5].
Case series of VAMPs reported a generally favorable clinical course with spontaneous resolution of symptoms during the first weeks and a very low rate of severe or life-threatening forms, similarly to viral myocarditis or pericarditis [6,7,8,9]. However, in some subjects myocardial inflammation could persist after months, requiring closer follow-up and specific therapies.
Characterizing clinical evolution of VAMPs and their correlation with imaging features of inflammation, would provide further insights into the clinical significance and prognostic determinants of this rare post-vaccine complication. This appears particularly necessary in order to answer the pressing request of data about the real safety profile of the vaccination campaign by public opinion.
Cardiac Magnetic Resonance (CMR) is widely considered as the reference non-invasive diagnostic option to confirm the diagnosis of VAMPs and to drive clinical decision-making and follow-up.
Our purpose was therefore to explore baseline and follow-up clinical and CMR features in a cohort of individuals with VAMPs recruited from a multicenter consortium of 13 national tertiary hospitals.
Methods
Study population
Our target population was retrospectively selected from a cohort of individuals presenting with cardiac symptoms, laboratory and imaging findings suggestive for an acute myocardial damage, within 25 days from COVID-19 vaccine injection, from 13 large tertiary Italian hospitals in the period from March 2021 to February 2022.
Our inclusion criteria were the following: (1) cardiac symptoms and/or ECG abnormalities, (2) troponin serum level increase, (3) evidence of active cardiac injury at a CMR examination performed within 20 days from symptom onset and 4) clinical and/or CMR follow-up of at least 30 days.
All patients had a confirmed diagnosis of myocarditis, pericarditis or myopericarditis as proposed by the Center for Disease Control and prevention [10, 11].
Patients with known pre-existing chronic conditions associated with myocardial inflammation (e.g. chronic myocarditis, rheumatic or autoimmune diseases, vasculitis) were excluded from the evaluation.
The study was approved by the institutional review board of the leading center. Informed consent was obtained from all the patients.
CMR protocols and findings
All demographic, clinical, laboratory and CMR data at baseline and short-term follow-up from all centers were collected, anonymized and analyzed by Sapienza University group.
Troponin serum level was considered increased if > 99th percentile than the normal range of each local laboratory standard.
All CMR protocols from different centers (Supplementary Table) included T2-weighted images, cine images and late gadolinium enhancement (LGE) sequences acquired after contrast media administration. In ten centers, T1 and/or T2 mapping sequences were also acquired. CMR images were evaluated by local radiologists with various years of experience in cardiac imaging (minimum 8 years). Differential diagnoses with alternative causes of myocardial injury were at the discretion of local physicians based on local assessments.
Left and right ventricular volumes and CMR features of tissue damage (presence of myocardial edema, LGE areas and abnormal myocardial T1 or T2 values) were collected in all patients. Left ventricular (LV) systolic function was categorized as normal if left ventricular ejection fraction (LVEF) was ≥ 50%; mildly decreased systolic function was defined with LVEF between 40 and 50%, moderately decreased if 30–40%, and severely decreased if < 30% [12].
According to the revised Lake Louise Criteria [13], active myocardial inflammation was defined by the presence of T1 criterion (LGE or native T1 increase or extracellular volume increase) and T2 criterion (edema in T2-weighted images or T2 ratio > 2 or T2 mapping increase). LGE and edema were visually assessed and categorized as “present” or “not present”; on both images, the distribution of LGE or edema was classified as subendocardial, mid-wall, subepicardial or transmural; the extent of the LGE was quantified as the number of segments involved according to the 16 segments model. Regional or global increase in T1 and T2 mapping values were defined when > 2 standard deviation (SD) above the local reference values, which vary for specific MR sequence/equipment, calculated from the local group based on a sample of healthy controls, as suggested by consensus document [14].
CMR signs of pericarditis, defined as thickening and enhancement of the pericardial layers with or without pericardial effusion, have been detected [15].
Clinical follow-up data were acquired with electronic medical records, follow-up visits or phone interviews.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (if normality could be assumed using the Shapiro-Wilks test) or median values with range. Independent variables were compared with unpaired t-test. Categorical variables, reported as counts and percentages, were arranged in cross-correlation tables and studied with the χ2 test or Fisher’s exact test. All the tests were 2-tailed, and only p values < 0.05 were considered statistically significant. Analysis was performed using SPSS software version 26.0 (IBM).
Results
Population and vaccination
A total of 44 patients were finally included in the study [females were 2 (4.5%)], with a mean age of 31.7 ± 15.1 years old. All the patients were aged ≥ 15 years old and 29 (65.9%) were < 35 years old. Patient selection flow-chart is reported on Fig. 1.
Mean time from the last vaccination dose to the onset of symptoms was 6.2 ± 5.6 days. The majority of the subjects included in the analysis received a vaccination with mRNA vaccine (Fig. 2) and predominantly after the second dose (Table 1).
Two patients had a history of previous Sars-CoV-2 infection (previous Sars-CoV-2 nasopharyngeal swab RT-PCR testing positivity), both at least six months earlier than the vaccine administration. One patient had a previous acute myocardial infarction. None presented with known valvular pathologies, stroke or tumors.
Baseline clinical and CMR features
Clinical, laboratory and CMR data are reported in Tables 1 and 2.
Chest pain was present in 41 patients (93.2%) who referred to the Emergency Department. Other symptoms included fever (29, 65.9%), myalgia (17, 38.6%), dyspnea (13, 29.5%) and palpitations (11, 25%). ECG anomalies (including ST segment elevation or depression, T-wave inversion, left or right bundle branch block and repolarization abnormalities) were found in 30 (68.1%).
Mean onset-to-CMR time was 6.8 ± 4 days. At CMR acquired during the acute phase, 5 (11.4%) patients presented with mild reduction of LVEF, whereas two patients (4.5%) had moderate LV function impairment and right ventricular systolic function was mildly reduced in 8 (18.2%) patients.
Regarding tissue characterization, edema was found in 35 (79.5%) and LGE in 40 (90.9%), both with a predominant subepicardial or mid-wall distribution pattern (80 and 97.5%, respectively), predominantly located in the mid-basal infero-lateral wall of the LV. Myocardial edema pattern was transmural in 7/35 (20%), whereas LGE was transmural in 1/40 (2.5%).
Mapping sequences were available in 35/44 patients for T1 and 34/44 patients for T2. T1 and T2 maps revealed regional or global increase of native T1 mapping in 24/35 (68.6%) patients and of T2 mapping in 28/34 (82.4%).
Diagnosis at discharge of active myocarditis was reached in 28 (63.6%) patients (Fig. 3); myo-pericarditis in 13 (29.5%) and pericarditis in 3 (6.8%) (Fig. 4).
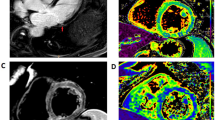
Vaccine-associated myocarditis. T2-weighted images (a) revealed the presence of myocardial edema located in the lateral wall of the left ventricle (arrowhead). A subepicardial LGE stria was evidenced on the same myocardial segments (b, white arrow). Those findings were confirmed by increased T1 (c) and T2 (d) mapping values on the lateral wall. At 117 days follow-up, CMR revealed a resolution of myocardial edema (e), with a reduction in LGE extension (f). Mapping sequences showed a decrease in T1 mapping values (g) and T2 values (h) that returned within the normal range
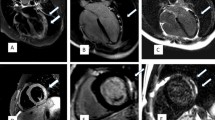
Vaccine-associated pericarditis. 57-year-old male admitted to ED for chest pain, fatigue and dyspnea after 8 days following second dose of Comirnaty vaccine. The first CMR (a–f), performed for the clinical suspicion of myocarditis, showed edematous thickening and enhancement of pericardial layers (red arrows) respectively on STIR T2-weighted (a) and fat-suppressed turbo spin echo T1-weighted (b) images. Absence of myocardial injury and pericardial enhancement (arrowheads) has been detected on late gadolinium enhanced images acquired on short axis (c) and horizontal long axis (d) view. Myocardial native T1 (e) and T2 (f) values were within normal range on corresponding maps. At 13-weeks follow-up CMR, there were neither pericardial fluid nor edema on STIR images (g) nor myocardial LGE areas (h)
Clinical and CMR follow-up
Clinical follow-up (FU) revealed absence of major events in all subjects and resolution of symptoms in most of them (82%). Persistent chest pain, myalgia, palpitations and dyspnea have been detected in 5 (11.4%), 2 (4.5%), 2 (4.5%) and 3 (6.8%) cases, respectively. ECG anomalies (repolarization abnormalities and left or right bundle branch block) were found in 5 (11.4%); none of the patients showed fever.
FU-CMR was available in 29 patients and revealed recovery of left ventricular function in 5 patients with reduced LVEF at baseline CMR (Fig. 5a) and persisting mild LVEF reduction in 2/29 (6.9%).
Mapping sequences were available in 16/29 patients for T1 and 21/29 patients for T2. Persistent signs of active myocardial inflammation were present in 8/29 (27.6%) patients, who showed an increase in T2 mapping values (8/29) and/or hyperintense areas on T2-weighted images (4/29). LGE was found in 26/29 (89.6%) patients, with a typical subepicardial or mid-layer distribution (Fig. 5b). Native T1 values were increased in 6 cases. Residual thickening and hyperintensity of the pericardial layers were found in just one patient, whereas pericardial effusion in 4/29 (13.8%). At CMR follow-up, signs of myocardial inflammatory activity on T2-weighted and mapping sequences or pericardial inflammation were significantly reduced (p = 0.033–0.001). Although there was no significant modification of LGE occurrence, the mean number of LGE + segments was reduced at follow-up (p: 0.016). Finally, no significant correlation was found between the presence of symptoms at clinical FU and the persistence of edema revealed by FU-CMR.
Discussion
Our study describes clinical and CMR characteristics of 44 patients, recruited from 13 tertiary national reference centers, that received a diagnosis of acute myocarditis, pericarditis or myo-pericarditis temporally related to COVID-19 vaccination, over a three-month follow-up period. To the best of our knowledge, our multicentric case collection included the largest patient cohort with CMR follow-up data reported in literature up to date.
Although complications related to COVID-19 vaccines are not fully explored, given their recent approval, VAMPs have been already extensively described as isolated cases or case series, especially when using mRNA vaccines [3, 7, 8, 16,17,18]. They represent a public safety concern, emphasized by the constant media attention to the worldwide massive vaccination campaign, and long-term implications need to be clarified.
As often pointed out, it should be specified that the temporal proximity between symptoms onset and vaccine inoculation is not per se sufficient to prove a causal link, and results of observational study on the general population should be considered cautiously. However, much evidences suggest that the vaccine may play a role as a trigger or a contributing cause of pericardial or myocardial inflammation in vaccinated patients [19,20,21,22]. Theoretically, the immune response induced by vaccination could also re-activate myocardial inflammation in subjects with recurrent myocarditis or chronic systemic inflammatory disease.
The first main finding emerged from our results is that VAMPs generally consist in a clinically uneventful syndrome with mild presentation and favorable outcome at follow-up. Second, CMR showed left ventricular functional recovery at short term follow-up in most of the cases, and significant reduction of signs of inflammation on CMR, which persisted in almost a quarter of the study population.
Our study population showed signs or symptoms of cardiac involvement with an average of 6.2 ± 5.6 days from the last dose of vaccine. According to the literature, the time interval between vaccination and cardiac symptoms may vary between 0 and 179 days [23], with most of the cases occurring within 7 days and a median time onset of 3 days [24]. Also, the first peak of clinical manifestation (within 1–3 days post vaccination) seems to be more associated with acute myocarditis; the second (between 15- and 30-days post-vaccination) with acute pericarditis [23].
In line with other reports [3, 7,8,9, 16,17,18,19], the most of our cases occurred using RNA-based vaccines. Anastassopoulou et al. [23] found Janssen and Vaxzevria to be more associated with cases of acute pericarditis, but in our cohort those vaccines both lead to myocarditis.
Furthermore, 95.5% of our patients were male and 65.9% were < 35 years old. The higher prevalence of acute myocardial injury among males has been attributed to the effects of sexual hormones on the immune response [19]: testosterone seems to lead to a greater T-lymphocytes activation rather than estrogens that stimulate inhibition of T cells [21]. As regards the more frequent involvement of young individuals, it has been hypothesized that it could be due to a stronger and reactive immune response as compared to older patients (higher levels of TNF alpha and IFN gamma in the youth as compared to the older age) [21]. Nevertheless, our cohort included a not negligible number of adults, thus VAMPs cannot be considered almost exclusively affecting the pediatric population.
In agreement with prior studies (Table 3), myocarditis, myopericarditis and pericarditis mostly presented as paucisymptomatic forms (chest pain, fever and dyspnea the most frequent symptoms) with preserved left ventricular function, generally self-limiting and with resolution of signs of active inflammation at the CMR short-time follow-up [25, 26].
Although in other series rare occurrence of intensive care unit admission (8.7%) and a mortality rate of 1.4% were reported [21], in our cohort no patient had severe or life-threatening conditions.
VAMPs seem to have a more benign prognosis as compared to myocarditis associated to COVID-19 with almost complete resolution of symptoms at short-term follow-up [27, 28]. Patone et al. [27] estimated the risk of developing acute myocarditis after the first or second dose of adenovirus or mRNA vaccination to be 1–10 per million, approximately; on the other hand, the risk of myocarditis following SARS-CoV-2 infection is placed around 40 per million.
Moreover, myocarditis represents only one of the potential cardiovascular complications related to Sars-Cov-2 infection [29,30,31] which are associated to increased risk of in-hospital mortality and worse prognosis at one-year follow-up [32], confirming the advantages of immunization in preventing cardiovascular diseases as compared to the risks associated with SARS-CoV-2 infection.
In line with previous studies [19, 22, 26, 33], the majority of our patients revealed clinical signs and symptoms of cardiac involvement after the second dose of mRNA vaccines (45.5%), even though the number of patients with onset after the first dose was consistent (40.9%).
Our CMR findings confirm those from Fronza et al. [26], which reported a low rate of LV systolic dysfunction and regional wall motion abnormalities in VAMPs patients as well as the prevalence and distribution pattern of myocardial edema and LGE (predominantly subepicardial and mid-wall). Those authors also demonstrated that CMR findings in vaccine-related myocarditis were similar to other forms with different etiologies, but with milder myocardial impairment (higher LV and RV EF and less extensive LGE were found in VAMP) [26].
A large meta-analysis of 102 studies, including a total of 468 patients with clinically suspected myocarditis following COVID-19 vaccination [34], demonstrated that left ventricular dysfunction is uncommon at clinical onset (LV-EF < 50%: 9.2%) and CMR signs of myocardial inflammation are frequent with rates very similar to those of our series (elevated nT1: 74.5%, T2 weighted or T2 STIR or T2 mapping abnormality: 81.9%; presence of LGE: 94%). Interestingly, pericardial enhancement was found in 32.8% of patients, confirming that pericardial involvement is a quite common feature in this condition.
The novel aspect of our study consists in the comparison between CMR acquired at baseline and at short-term follow-up. Most of the patients improved their LV and RV systolic function at FU and signs of active inflammation were still evident in 8/29 patients. Residual LGE was found in 26/29, reflecting the fibrotic evolution of myocardial damage, which has been associated with an increased risk of major cardiovascular events [35, 36]. It is known that in patients with viral myocarditis, the presence of LGE with anterior or septal midwall LGE is correlated to a greater mortality rate as compared with other LGE distribution patterns or with the complete absence of LGE [37]. In our study population, most of the patients showed an infero-lateral LGE location, suggesting a more favorable outcome; but the real prognostic impact of LGE in this population should be investigated with long-term follow-up studies with larger cohorts.
At short-term CMR-FU, VAMPs showed similar features as compared to viral myocarditis: persistence of LGE, progressive resolution of myocardial edema on T2 weighted images [38] and decrease of T1 and T2 mapping values [38]. As regards LVEF, a study by Ammirati et al. [39] conducted on 76 patients with classical acute myocarditis revealed the increase of systolic function at a median follow-up of 148 days in whom had a baseline EF < 55% [39].
The similarity of CMR features between VAMPs and other forms of myocarditis likely reflects analogous pathophysiological mechanisms. Several pathways of myocardial injury have been hypothesized following COVID-19 vaccination. Firstly, the so-called “molecular mimicry” theory, in which the immune cross-reactivity between the viral antigen and myocardial proteins (e.g. alpha-myosin) is induced [19, 20], that could justify the prevalence of acute myocarditis after the second dose [24]. Another hypothesis considers the development of an inflammatory response against the mRNA detected as an antigen by the immune system [21]. Finally, the viral surface protein seems to interact with angiotensin converting enzyme 2 receptors, stimulating the immune system activation and cardiac sensitivity [22].
General agreement converges on a transient dysregulated immune response, also supported by the evidence at endomyocardial biopsy of mixed inflammatory infiltrates with acute lymphocytic myocarditis [24] or degranulated eosinophils consistent with a pattern of hypersensitivity myocarditis [40].
Study limitations
The study is retrospective and the analysis collects data from several hospitals, where both clinical assessment and diagnostic examinations were subject to the local physicians’ decisions. CMR were performed with different scanners and protocols. In particular, T1 or T2 mapping sequences were not always available or performed and this could have affected diagnostic performance of CMR in those centers, resulting in lower sensitivity for cases with subtle or diffuse myocardial damage. Moreover, our cohort included only patients with CMR findings consistent with diagnosis of myocarditis and/or pericarditis, therefore it is likely that cases of minimal vaccine-related myocardial injury, not detectable by CMR, could have been excluded from the enrollment. Furthermore, in patients scanned at follow-up without mapping sequences, we cannot exclude that mild active inflammation persisted even in the absence of areas of hyperintensity on edema-weighted sequences. Another limitation is the non-negligible number of patients who did not perform clinical (13/57, 22.8%) or CMR (15/44, 34%) follow-up, even though the number of subjects who completed the assessment was sufficient to perform the analysis. Histological confirmation, actually still the gold standard for diagnosing acute myocarditis, was not obtained in our entire patients’ population and therefore diagnosis of myocarditis was based on clinical and CMR findings in most of the cases.
The definition of VAMPs relied on the temporal association between COVID-19 vaccination and the onset of symptoms and we are not able to exclude other causes of myocarditis or pericarditis.
Conclusion
Acute myocarditis, pericarditis or myopericarditis following COVID-19 vaccination are generally characterized by mild clinical presentation with typical CMR features of myocardial and/or pericardial inflammation. Short-term follow-up demonstrated self-limiting course and resolution of CMR signs of active inflammation in most of the cases. Further studies with larger case series and longer follow-up are required to better understand the characteristics of this syndrome, long-term outcomes and to depict its peculiarities with respect to the other forms of myocarditis and pericarditis.
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- FU:
-
Follow-up
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- RV:
-
Right ventricle
- VAMP:
-
Vaccine-associated myocarditis and pericarditis
References
Hannah Ritchie, Edouard Mathieu, Lucas Rodés-Guirao, Cameron Appel, Charlie Giattino, Esteban Ortiz-Ospina, Joe Hasell, Bobbie Macdonald DB and MR Coronavirus Pandemic (COVID-19)
Di Dedda EA, Barison A, Aquaro GD et al (2022) Cardiac magnetic resonance imaging of myocarditis and pericarditis following COVID-19 vaccination: a multicenter collection of 27 cases. Eur Radiol 32:4352–4360. https://doi.org/10.1007/s00330-022-08566-0
Truong DT, Dionne A, Muniz JC et al (2022) Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation 145:345–356. https://doi.org/10.1161/CIRCULATIONAHA.121.056583
Moroni F, Mbualungu J, Abbate A (2022) Myocarditis after RNA-based COVID-19 vaccines: where do we stand? Int J Cardiol 356:81–82. https://doi.org/10.1016/j.ijcard.2022.03.014
Chin SE, Bhavsar SM, Corson A et al (2022) Cardiac Complications Associated with COVID-19, MIS-C, and mRNA COVID-19 Vaccination. Pediatr Cardiol 43:483–488. https://doi.org/10.1007/s00246-022-02851-x
Abbate A, Gavin J, Madanchi N et al (2021) Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int J Cardiol 340:119–121. https://doi.org/10.1016/j.ijcard.2021.08.018
Jain SS, Steele JM, Fonseca B et al (2021) COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. https://doi.org/10.1542/peds.2021-053427
Puchalski M, Kamińska H, Bartoszek M et al (2022) COVID-19-vaccination-induced myocarditis in teenagers: case series with further follow-up. Int J Environ Res Public Health 19:3456. https://doi.org/10.3390/ijerph19063456
Manfredi R, Bianco F, Bucciarelli V et al (2022) Clinical profiles and CMR findings of young adults and pediatrics with acute myocarditis following mRNA COVID-19 vaccination: a case series. Vaccines 10:169. https://doi.org/10.3390/vaccines10020169
(CDC) C for DC and P (2021) Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices
Gargano JW, Wallace M, Hadler SC et al (2021) Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices — United States, June 2021. MMWR Morb Mortal Wkly Rep 70:977–982. https://doi.org/10.15585/mmwr.mm7027e2
Di Cesare E, Carerj S, Palmisano A et al (2021) Multimodality imaging in chronic heart failure. Radiol Med 126:231–242. https://doi.org/10.1007/s11547-020-01245-4
Ferreira VM, Schulz-Menger J, Holmvang G et al (2018) Cardiovascular magnetic resonance in nonischemic myocardial inflammation. J Am Coll Cardiol 72:3158–3176. https://doi.org/10.1016/j.jacc.2018.09.072
Messroghli DR, Moon JC, Ferreira VM et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the society for cardiovascular magnetic resonance (SCMR) endorsed by the European association for cardiovascular imagi. J Cardiovasc Magn Reson 19:75. https://doi.org/10.1186/s12968-017-0389-8
Bogaert J, Francone M (2013) Pericardial disease: value of CT and MR imaging. Radiology 267:340–356. https://doi.org/10.1148/radiol.13121059
Das BB, Kohli U, Ramachandran P et al (2021) Myopericarditis after messenger RNA coronavirus disease 2019 vaccination in adolescents 12 to 18 years of age. J Pediatr 238:26-32.e1. https://doi.org/10.1016/j.jpeds.2021.07.044
Dionne A, Sperotto F, Chamberlain S et al (2021) Association of myocarditis With BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol 6:1446. https://doi.org/10.1001/jamacardio.2021.3471
Amir G, Rotstein A, Razon Y et al (2022) CMR imaging 6 months after myocarditis associated with the BNT162b2 mRNA COVID-19 vaccine. Pediatr Cardiol. https://doi.org/10.1007/s00246-022-02878-0
Fatima M, Ahmad Cheema H, Ahmed Khan MH et al (2022) Development of myocarditis and pericarditis after COVID-19 vaccination in adult population: a systematic review. Ann Med Surg 76:103486. https://doi.org/10.1016/j.amsu.2022.103486
Saeed S, Käsk L, Rajani R, Larsen TH (2022) Incidence, clinical presentation, and management of myocarditis following mRNA-based COVID -19 vaccines: a brief report. Cardiology. https://doi.org/10.1159/000522216
Goyal M, Ray I, Mascarenhas D et al (2022) Myocarditis post-SARS-CoV-2 vaccination: a systematic review. QJM An Int J Med. https://doi.org/10.1093/qjmed/hcac064
Ilonze OJ, Guglin ME (2022) Myocarditis following COVID-19 vaccination in adolescents and adults: a cumulative experience of 2021. Heart Fail Rev. https://doi.org/10.1007/s10741-022-10243-9
Anastassopoulou C, Hatziantoniou S, Vlachopoulos C et al (2022) Temporal relationship of myocarditis and pericarditis following COVID-19 vaccination: a pragmatic approach. Int J Cardiol 358:136–139. https://doi.org/10.1016/j.ijcard.2022.04.024
Lee ASY, Balakrishnan IDD, Khoo CY et al (2022) Myocarditis following COVID-19 vaccination: a systematic review (October 2020–October 2021). Hear Lung Circ 31:757–765. https://doi.org/10.1016/j.hlc.2022.02.002
Shiyovich A, Witberg G, Aviv Y et al (2021) Myocarditis following COVID-19 vaccination: magnetic resonance imaging study. Eur Hear J - Cardiovasc Imaging. https://doi.org/10.1093/ehjci/jeab230
Fronza M, Thavendiranathan P, Karur GR et al (2022) Cardiac MRI and clinical follow-up in COVID-19 vaccine–associated myocarditis. Radiology. https://doi.org/10.1148/radiol.220802
Patone M, Mei XW, Handunnetthi L et al (2022) Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 28:410–422. https://doi.org/10.1038/s41591-021-01630-0
Kravchenko D, Isaak A, Mesropyan N et al (2022) Cardiac magnetic resonance follow-up of COVID-19 vaccine associated acute myocarditis. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2022.1049256
Catapano F, Marchitelli L, Cundari G et al (2021) Role of advanced imaging in COVID-19 cardiovascular complications. Insights Imaging 12:28. https://doi.org/10.1186/s13244-021-00973-z
Esposito A, Palmisano A, Natale L et al (2020) Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in Covid-19. JACC Cardiovasc Imaging 13:2462–2465. https://doi.org/10.1016/j.jcmg.2020.06.003
Galea N, Catapano F, Marchitelli L et al (2021) How to perform a cardio-thoracic magnetic resonance imaging in COVID-19: comprehensive assessment of heart, pulmonary arteries, and lung parenchyma. Eur Hear J- Cardiovasc Imaging 22:728–731. https://doi.org/10.1093/ehjci/jeaa335
Maestrini V, Birtolo LI, Francone M et al (2021) Cardiac involvement in consecutive unselected hospitalized COVID-19 population: In-hospital evaluation and one-year follow-up. Int J Cardiol 339:235–242. https://doi.org/10.1016/j.ijcard.2021.06.056
Holland DJ, Blazak PL, Martin J et al (2022) Myocarditis and cardiac complications associated with COVID-19 and mRNA vaccination: a pragmatic narrative review to guide clinical practice. Hear Lung Circ 31:924–933. https://doi.org/10.1016/j.hlc.2022.03.003
Samimisedeh P, Jafari Afshar E, Shafiabadi Hassani N, Rastad H (2022) Cardiac MRI findings in COVID-19 vaccine-related myocarditis: a pooled analysis of 468 patients. J Magn Reson Imaging 56:971–982. https://doi.org/10.1002/jmri.28268
Hadley SM, Prakash A, Baker AL et al (2022) Follow-up cardiac magnetic resonance in children with vaccine-associated myocarditis. Eur J Pediatr 181:2879–2883. https://doi.org/10.1007/s00431-022-04482-z
Bohbot Y, Garot J, Hovasse T et al (2022) Clinical and cardiovascular magnetic resonance predictors of early and long-term clinical outcome in acute myocarditis. Front Cardiovasc Med. https://doi.org/10.3389/fcvm.2022.886607
Greulich S, Seitz A, Müller KAL et al (2020) Predictors of mortality in patients with biopsy-proven viral myocarditis: 10-Year outcome data. J Am Heart Assoc. https://doi.org/10.1161/JAHA.119.015351
von Knobelsdorff-Brenkenhoff F, Schüler J, Dogangüzel S et al (2017) Detection and monitoring of acute myocarditis applying quantitative cardiovascular magnetic resonance. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.116.005242
Ammirati E, Moroni F, Sormani P et al (2017) Quantitative changes in late gadolinium enhancement at cardiac magnetic resonance in the early phase of acute myocarditis. Int J Cardiol 231:216–221. https://doi.org/10.1016/j.ijcard.2016.11.282
Frustaci A, Verardo R, Galea N et al (2022) Hypersensitivity myocarditis after COVID-19 mRNA vaccination. J Clin Med 11:1660. https://doi.org/10.3390/jcm11061660
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
NG, MF and GC: participated in study concept and design. GC, EDD, CrCh, GDA, AB, RC, EDC, PDR, AE, RF, MG, CL, LL, CM, CBM, AP, SP, FR, LS, FS, CaCa and MF: were involved in data collection and participated in the data analysis. NG and GC: performed data analysis and statistical analysis. EDD: contributed to data interpretation. NG and GC drafted the manuscript. MF, CM, SP and MG: were involved in the critical revision of the manuscript. All authors read and approved the final manuscript. NG is the corresponding author and is responsible for the content of the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Ethical approval
The study has been approved by the local ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All enrolled subjects gave their informed consent prior to their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galea, N., Cundari, G., Di Dedda, E. et al. Short term outcome of myocarditis and pericarditis following COVID-19 vaccines: a cardiac magnetic resonance imaging study. Int J Cardiovasc Imaging 39, 1031–1043 (2023). https://doi.org/10.1007/s10554-023-02799-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02799-w