Abstract
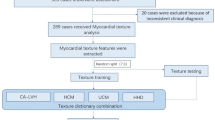
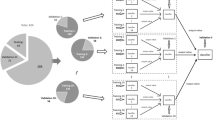
Cardiac amyloidosis has a poor prognosis, and high mortality and is often misdiagnosed as hypertrophic cardiomyopathy, leading to delayed diagnosis. Machine learning combined with speckle tracking echocardiography was proposed to automate differentiating two conditions. A total of 74 patients with pathologically confirmed monoclonal immunoglobulin light chain cardiac amyloidosis and 64 patients with hypertrophic cardiomyopathy were enrolled from June 2015 to November 2018. Machine learning models utilizing traditional and advanced algorithms were established and determined the most significant predictors. The performance was evaluated by the receiver operating characteristic curve (ROC) and the area under the curve (AUC). With clinical and echocardiography data, all models showed great discriminative performance (AUC > 0.9). Compared with logistic regression (AUC 0.91), machine learning such as support vector machine (AUC 0.95, p = 0.477), random forest (AUC 0.97, p = 0.301) and gradient boosting machine (AUC 0.98, p = 0.230) demonstrated similar capability to distinguish cardiac amyloidosis and hypertrophic cardiomyopathy. With speckle tracking echocardiography, the predictive performance of the voting model was similar to that of LightGBM (AUC was 0.86 for both), while the AUC of XGBoost was slightly lower (AUC 0.84). In fivefold cross-validation, the voting model was more robust globally and superior to the single model in some test sets. Data-driven machine learning had shown admirable performance in differentiating two conditions and could automatically integrate abundant variables to identify the most discriminating predictors without making preassumptions. In the era of big data, automated machine learning will help to identify patients with cardiac amyloidosis and timely and effectively intervene, thus improving the outcome.




Similar content being viewed by others
References
Falk RH, Alexander KM, Liao R et al (2016) AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 68(12):1323–1341. https://doi.org/10.1016/j.jacc.2016.06.053
Ruberg FL, Grogan M, Hanna M et al (2019) Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 73(22):2872–2891. https://doi.org/10.1016/j.jacc.2019.04.003
Wechalekar AD, Gillmore JD, Hawkins PN (2016) Systemic amyloidosis. Lancet (London, England) 387(10038):2641–2654. https://doi.org/10.1016/s0140-6736(15)01274-x
Phelan D, Collier P, Thavendiranathan P et al (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98(19):1442–1448. https://doi.org/10.1136/heartjnl-2012-302353
Motwani M, Dey D, Berman DS et al (2017) Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J 38(7):500–507. https://doi.org/10.1093/eurheartj/ehw188
Sanchez-Martinez S, Duchateau N, Erdei T et al (2018) Machine learning analysis of left ventricular function to characterize heart failure with preserved ejection fraction. Circ Cardiovasc Imaging 11(4):e007138. https://doi.org/10.1161/circimaging.117.007138
Narula S, Shameer K, Salem Omar AM et al (2016) Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol 68(21):2287–2295. https://doi.org/10.1016/j.jacc.2016.08.062
Sengupta PP, Huang YM, Bansal M et al (2016) Cognitive machine-learning algorithm for cardiac imaging: a pilot study for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ Cardiovasc Imaging. https://doi.org/10.1161/circimaging.115.004330
Awan SE, Bennamoun M, Sohel F et al (2019) Machine learning-based prediction of heart failure readmission or death: implications of choosing the right model and the right metrics. ESC Heart Fail 6(2):428–435. https://doi.org/10.1002/ehf2.12419
Attia ZI, Noseworthy PA, Lopez-Jimenez F et al (2019) An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet (London, England) 394(10201):861–867. https://doi.org/10.1016/s0140-6736(19)31721-0
Raj S, Ray KC (2018) A personalized arrhythmia monitoring platform. Sci Rep 8(1):11395. https://doi.org/10.1038/s41598-018-29690-2
Dorbala S, Ando Y, Bokhari S et al (2019) ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2-evidence base and standardized methods of imaging. J Card Fail 25(11):e1–e39. https://doi.org/10.1016/j.cardfail.2019.08.001
Ommen SR, Mital S, Burke MA et al (2020) 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation 142(25):e533–e557. https://doi.org/10.1161/cir.0000000000000938
Churpek MM, Yuen TC, Winslow C et al (2016) Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med 44(2):368–374. https://doi.org/10.1097/ccm.0000000000001571
Mortazavi BJ, Bucholz EM, Desai NR et al (2019) Comparison of machine learning methods with national cardiovascular data registry models for prediction of risk of bleeding after percutaneous coronary intervention. JAMA Netw Open 2(7):e196835. https://doi.org/10.1001/jamanetworkopen.2019.6835
Al’Aref SJ, Singh G, van Rosendael AR et al (2019) Determinants of in-hospital mortality after percutaneous coronary intervention: a machine learning approach. J Am Heart Assoc 8(5):e011160. https://doi.org/10.1161/jaha.118.011160
Sun JP, Stewart WJ, Yang XS et al (2009) Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol 103(3):411–415. https://doi.org/10.1016/j.amjcard.2008.09.102
Di Bella G, Minutoli F, Pingitore A et al (2011) Endocardial and epicardial deformations in cardiac amyloidosis and hypertrophic cardiomyopathy. Circ J 75(5):1200–1208. https://doi.org/10.1253/circj.cj-10-0844
Baccouche H, Maunz M, Beck T et al (2012) Differentiating cardiac amyloidosis and hypertrophic cardiomyopathy by use of three-dimensional speckle tracking echocardiography. Echocardiography 29(6):668–677. https://doi.org/10.1111/j.1540-8175.2012.01680.x
Liu D, Hu K, Niemann M et al (2013) Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging 6(6):1066–1072. https://doi.org/10.1161/circimaging.113.000683
Pagourelias ED, Mirea O, Duchenne J et al (2017) Echo parameters for differential diagnosis in cardiac amyloidosis: a head-to-head comparison of deformation and nondeformation parameters. Circ Cardiovasc Imaging 10(3):e005588. https://doi.org/10.1161/circimaging.116.005588
Boldrini M, Cappelli F, Chacko L et al (2020) Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging 13(4):909–920. https://doi.org/10.1016/j.jcmg.2019.10.011
Zhang J, Gajjala S, Agrawal P et al (2018) Fully automated echocardiogram interpretation in clinical practice. Circulation 138(16):1623–1635. https://doi.org/10.1161/circulationaha.118.034338
Shameer K, Johnson KW, Glicksberg BS et al (2018) Machine learning in cardiovascular medicine: are we there yet? Heart 104(14):1156–1164. https://doi.org/10.1136/heartjnl-2017-311198
Acknowledgements
The authors thank Xiao-Jun Chen for providing purely technical help.
Funding
This research did not receive any specific grant from funding: agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ZW as the first author collected data, drafted, and revised the manuscript. JZ and LK contributed to the acquisition, analysis, and interpretation of data. HY is the corresponding author, who performed the conception and design of the study, and the final approval of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
All of the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. IIT20200654A).
Consent for publication
Consent for publication was obtained for every individual person’s data included in the study.
Informed consent
Informed consent was obtained from all of the individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, ZW., Zheng, JL., Kuang, L. et al. Machine learning algorithms to automate differentiating cardiac amyloidosis from hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 39, 339–348 (2023). https://doi.org/10.1007/s10554-022-02738-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02738-1