Abstract
Cardiac involvement has been described in varying proportions of patients recovered from COVID-19 and proposed as a potential cause of prolonged symptoms, often described as post-COVID or long COVID syndrome. Recently, cardiac complications have been reported from COVID-19 vaccines as well. We aimed to compare CMR-findings in patients with clinical cardiac symptoms after COVID-19 and after vaccination. From May 2020 to May 2021, we included 104 patients with suspected cardiac involvement after COVID-19 who received a clinically indicated cardiac magnetic resonance (CMR) examination at a high-volume center. The mean time from first positive PCR to CMR was 112 ± 76 days. During their COVID-19 disease, 21% of patients required hospitalization, 17% supplemental oxygen and 7% mechanical ventilation. In 34 (32.7%) of patients, CMR provided a clinically relevant diagnosis: Isolated pericarditis in 10 (9.6%), %), acute myocarditis (both LLC) in 7 (6.7%), possible myocarditis (one LLC) in 5 (4.8%), ischemia in 4 (3.8%), recent infarction in 2 (1.9%), old infarction in 4 (3.8%), dilated cardiomyopathy in 3 (2.9%), hypertrophic cardiomyopathy in 2 (1.9%), aortic stenosis, pleural tumor and mitral valve prolapse each in 1 (1.0%). Between May 2021 and August 2021, we examined an additional 27 patients with suspected cardiac disease after COVID-19 vaccination. Of these, CMR provided at least one diagnosis in 22 (81.5%): Isolated pericarditis in 4 (14.8%), acute myocarditis in 9 (33.3%), possible myocarditis (acute or subsided) in 6 (22.2%), ischemia in 3 (37.5% out of 8 patients with stress test), isolated pericardial effusion (> 10 mm) and non-compaction-cardiomyopathy each in 1 (3.7%). The number of myocarditis diagnoses after COVID-19 was highly dependent on the stringency of the myocarditis criteria applied. When including only cases of matching edema and LGE and excluding findings in the right ventricular insertion site, the number of cases dropped from 7 to 2 while the number of cases after COVID-19 vaccination remained unchanged at 9. While myocarditis is an overall rare side effect after COVID-19 vaccination, it is currently the leading cause of myocarditis in our institution due to the large number of vaccinations applied over the last months. Contrary to myocarditis after vaccination, LGE and edema in myocarditis after COVID-19 often did not match or were confined to the RV-insertion site. Whether these cases truly represent myocarditis or a different pathological entity is to be determined in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cardiac complications during the acute phase of COVID-19
The SARS-CoV-2 epidemic has strained the resources of health care systems and judicious use of advanced imaging modalities is necessary to prevent overburdening. While the primary target of SARS-CoV-2 is the respiratory system, cardiac complications have been reported in varying frequency [1]. An early observational study from Wuhan reported troponin I elevations in 37.5% and clinically suspected myocarditis in 12.5% of hospitalized COVID-19 patients based on symptoms, electrocardiography (ECG) and echocardiography [2]. Subsequent histopathological studies found any cardiac pathology in 47.8% of deceased patients but myocarditis in less than 2% [3]. Other reported findings were pericarditis, macro- and microthrombi, “nonmyocarditis” inflammation and acute myocardial infarction [3, 4].
Persistent cardiopulmonary symptoms after COVID-19
Persistent cardiopulmonary symptoms after the acute phase of COVID-19 are common. In a study of 143 hospitalized COVID-19 patients in Italy, 86.4% were still symptomatic at 2 months after symptom onset [5]. Common complaints were fatigue, dyspnea and chest pain. Another study of 1733 hospitalized patients in Wuhan in early 2020 with a median follow up of 6 months showed persistent fatigue in 63%, palpitations in 9% and chest pain in 5% of patients [6]. A prospective study of 247 Norwegian home-isolated patients found persistent fatigue (30%), dyspnea (15%) and palpitations (6%) at 6 months follow up [7]. The British National Institute for Health and Care Excellence guideline on the managing of the long-term effects of COVID-19 recommends the term “ongoing symptomatic COVID-19” for symptoms 4 to 12 weeks and “post-COVID-19 syndrome” for symptoms more than 12 weeks after the start of acute COVID-19 [8]. Another commonly used term is “long COVID” [7].
Cardiac magnetic resonance (CMR) in COVID-19
CMR offers the non-invasive assessment of several cardiac pathologies including myocarditis, pericarditis and embolic complications. CMR studies in patients recovered from the acute phase of COVID-19 have reported widely differing proportions of myocarditis after COVID-19 ranging from 0 to 60% of patients owing mostly to different diagnostic criteria and patient selection [1]. The International Consensus Group on CMR Diagnosis of Myocarditis published a white paper in 2009, proposing three “Lake Louise Criteria” (LCC) consisting of edema, early enhancement and LGE, two of which were required for a CMR-diagnosis of myocarditis [9]. A 2018 update of those criteria dropped early enhancement and incorporated T1-, ECV and T2-mapping-techniques as alternative parameters of fibrosis and edema, with one fibrosis- and one edema-criterion required for a CMR-diagnosis of myocarditis [10]. Most studies published so far have not consistently applied these criteria, rendering comparisons challenging [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Moreover, most published studies have examined competitive athletes, which are not representative of the patient collective in daily clinical practice [11, 13,14,15, 19, 20, 22,23,24]. To date there are only small case series on the prevalence of myocarditis in non-athletes which consistently applied the updated Lake-Louise-criteria. Weckbach et al. examined 18 patients with elevated troponin, seven of which had myocarditis [25]. Huang et al. examined 26 patients with cardiac symptoms, seven of which had myocarditis [16]. A study by Chen et al. in 25 acute hospitalized COVID-19 patients found myocardial edema in 14 and myocardial damage in one patient [12]. A study in 18 asymptomatic pediatric patients after mild COVID-19 found no apparent cardiac involvement by CMR [26].
Cardiac complications after COVID-19-vaccination
Since early 2021, reports of myocarditis after COVID-19-Vaccination have surfaced, mostly considered ‘mild’ with rapid clinical improvement [27,28,29,30,31,32,33,34]. Unless stated otherwise, ´vaccination´ in this paper denominates COVID-19-vaccination. Case-series reported resolution of edema and reduction of the size of LGE on follow-up CMR [35, 36]. While the pathophysiologic mechanism remains unclear and the number of cases is low compared to the number of vaccinations, the close temporal correlation with higher than expected absolute case numbers suggests a causal connection [37, 38].
Aim of the current study
We aimed to examine the prevalence of cardiac findings on CMR in a representative clinical collective of patients referred for suspected cardiac involvement after COVID-19 and vaccination.
Methods
We searched our hospital database for patients that underwent a clinical CMR examination between May 2020 and May 2021 for suspected cardiac pathology after COVID-19. For comparison, CMR examinations from January until August 2021 were searched for suspected cardiac pathology after COVID-19 vaccination. In case of multiple COVID-19 vaccinations, the last vaccination preceding symptoms was suspected as the causative vaccination. The study complies with the declaration of Helsinki and was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA2/020/21) with a waiver of consent. It is registered at ClinicalTrials.gov (NCT05124223). A subset of the patients was published earlier as case series [34, 39].
CMR imaging
All scans were performed for clinical indications on either a Philips Ingenia 3.0T scanner or a Philips Ambition 1.5T scanner (Koninklike Philips N.V., Amsterdam, The Netherlands) according to recent recommendations [1, 10, 40]. Protocols were adjusted to the clinical scenario but generally included standard CINE imaging, T2 STIR edema imaging, T2 mapping (T2-GraSE), pre- and post-contrast T1 mapping (MOLLI), and Late-Enhancement-Imaging (mDIXON) [39]. Vasodilator stress with Regadenosone or Adenosine was performed as clinically indicated in patients with suspected myocardial ischemia. The contrast agent dose was 0.1–0.15 mmol/kg Gadobutrol (Gadovist®, Bayer AG, Leverkusen, Germany) [41].
CMR image analysis
Image post-processing and measurements were performed according to recent recommendations using dedicated CMR post-processing software (IntelliSpace Portal V11.1, Koninklike Philips N.V., Amsterdam, The Netherlands) [42]. The diagnosis of acute myocarditis was based on the updated Lake Louise Criteria (LLC) requiring findings of myocardial damage (non-ischemic LGE) and edema (T2 STIR or T2 mapping) in a non-ischemic pattern (intramyocardial or subepicardial). Patients fulfilling only one LLC criterion were considered possible myocarditis, further divided into edema without myocardial damage as ‘possible acute myocarditis’ and myocardial damage without edema as ‘possible subsided myocarditis’.
Statistical analysis
Statistical analysis was performed using SPSS 25 (IBM, Armonk, NY, USA) and RStudio (RStudio PBC, Boston, MA, USA). Baseline data were reported as means ± standard deviations (SD) for interval- and ratio-scaled parameters and as numbers and percentages for nominal and ordinal-scaled parameters.
Results
Baseline characteristics
The baseline characteristics of post-COVID-19 and post-vaccination patients are given in Table 1. About four times more patients were referred for suspected cardiac involvement after COVID-19 than after vaccination. Post-COVID-19 patients were slightly older (47.6 ± 14.0 vs. 43.9 ± 20.4 years) and had more comorbidities than post-vaccination patients. Post-vaccination patients more often complained of cardiac symptoms at the time of CMR compared to post-COVID-19 patients (92.6% vs. 55.8%) and more frequently presented with elevated troponin (42.1 vs. 27.0%), although data on the latter was missing in many post-COVID-patients. The time from suspected causative event to CMR was longer in the post-COVID-19 patients (time from first positive PCR to CMR, 112 ± 76 days) than in post-vaccination patients (time from vaccine to CMR, 44 ± 35 days).
CMR results
CMR results are presented in Table 2 and the final CMR diagnoses in Table 3. The majority of post-COVID-19 patients were scanned on a 3 Tesla scanner whereas post-vaccination patients were scanned equally on 1.5 and 3 Tesla scanners. While the number of patients with suspected cardiac involvement after vaccination was smaller than after COVID-19, the prevalence of findings in patients after vaccination was higher. In 34 (32.7%) of post-COVID-19 patients, CMR provided a clinically relevant diagnosis: Isolated pericarditis in 10 (9.6%), acute myocarditis (both LLC) in 7 (6.7%), possible myocarditis (one LLC) in 5 (4.8%), ischemia in 4 (3.8%), recent infarction in 2 (1.9%), old infarction in 4 (3.8%), dilated cardiomyopathy in 3 (2.9%), hypertrophic cardiomyopathy in 2 (1.9%), aortic stenosis, pleural tumor and mitral valve prolapse each in 1 (1.0%). In post-vaccination patients, CMR provided at least one diagnosis in 22 (81.5%): Isolated pericarditis in 4 (14.8%), acute myocarditis in 9 (33.3%), possible myocarditis (acute or subsided) in 6 (22.2%), ischemia in 3 (37.5% out of 8 patients with stress test), isolated pericardial effusion (> 10 mm) and non-compaction-cardiomyopathy each in 1 (3.7%).
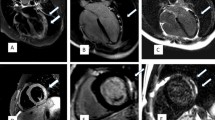
The clinical characteristics of all patients with acute myocarditis or possible acute myocarditis are listed in Table 4 for post-COVID-19 and in Table 5 for post-vaccination patients. In all 13 Patients with acute myocarditis on CMR and data on ECG, echocardiography and troponin, at least one of the latter was abnormal. Figures 1 and 2 show exemplary CMR images of post-COVID-19 patients and post-vaccination patients with probable acute myocarditis.
CMR images of a patient with myocarditis post-COVID (patient C5). A 3 chamber view, late gadolinium enhancement shows a subepicardial fibrosis inferolateral basal (arrow). B Medial short axis, T1 mapping shows no evidence of diffuse fibrosis. C Basal short axis, T2 STIR imaging showed edema at the inferior RV-insertion-site (arrow). D Medial short axis, T2 mapping shows no evidence of global edema
CMR images of a patient with perimyocarditis post COVID-Vaccine (Patient V1). All images medial short axis. A Late gadolinium enhancement shows subepicardial fibrosis with pericardial involvement. B T1 mapping shows focal but not diffuse fibrosis. C T2 STIR imaging shows subepicardial and pericardial edema (arrows). D T2 mapping shows no evidence of global edema
Time course of myocarditis cases
Quarterly cases of probable acute myocarditis and subsided myocarditis post COVID-19 and post vaccination are shown in Fig. 3a. For context, the monthly numbers of COVID-19-cases and vaccinations are shown in Fig. 3b [43, 44].
Discussion
Summary of findings
We analyzed 104 patients after COVID-19 and 27 patients after COVID-19-vaccination referred for CMR due to suspected cardiac involvement. The most frequent findings were
-
Post COVID-19: 10 (8.7%) isolated pericarditis, 7 (6.7%) acute myocarditis (both LLC), 5 (4.8%) possible myocarditis (one LLC), 4 Ischemia (6.3% out of 63 with stress test) and 4 (3.8%) old myocardial infarction (all in patients with known coronary artery disease).
-
Post vaccination: 9 (33.3%) acute myocarditis (both LLC), 6 (22.2%) possible myocarditis (one LCC), 4 (14.8%) isolated pericarditis and 3 (37.5% of 8 with stress test) ischemia.
These numbers are to be interpreted against the backdrop of 178,223 COVID-19 cases (per May 31th 2021) and 4,480,385 vaccination doses administered (per August 31th 2021) in Berlin (Fig. 3).
Interpretation
COVID-19 causes cardiac symptoms in a substantial number of patients, explaining the higher number of referred patients post-COVID-19 compared to post-vaccination. Unfortunately, this referral did not lead to a specific diagnosis in the majority of cases after COVID-19, underlining our incomplete understanding of long COVID. The absolute number of myocarditis and pericarditis diagnoses was similar after COVID-19 and after vaccination, despite the far lower number of COVID-19 cases than vaccinations in Berlin. For perspective, the total number of myocarditis cases examined by CMR at our institution in 2019 was 17. Compared to myocarditis cases after vaccination, myocarditis cases after COVID-19 showed a less typical appearance. Localized, matching edema and LGE excluding the inferoseptal insertion point was present in only two of the seven cases after COVID-19 compared to nine of nine cases after vaccination. All cases of CMR diagnosed acute myocarditis had abnormalities on either ECG, echocardiography or troponin, when those were available.
Comparison with other studies
Our findings are in line with those of other groups using the same diagnostic criteria and patients with clinical indication for CMR post-COVID-19 (Table 6) [11, 13,14,15,16,17,18,19,20,21,22,23,24,25]. The largest study to date, a retrospective analysis of 1597 screening-CMRs performed in athletes, reported CMR findings consistent with myocarditis in 37 (2.3%) participants. Seventeen (1.1% of total) of these had clinical suspicion of myocarditis based on symptoms or cardiac screening tests and 12 (0.8% of total) of those fulfilled both updated Lake-Louise-criteria [14].
The frequency of pericarditis was inconsistently reported in published CMR studies post COVID-19. Pericardial findings on CMR possibly associated with pericarditis include effusion, thickening, inspiratory septum shift, edema on T2 weighted imaging, and LGE [45]. Current guideline-based diagnostic criteria for pericarditis treat CMR only as adjunctive evidence and no universally agreed-upon CMR criteria for pericarditis exist [46]. In our experience slight pericardial LGE is very common and by itself not suggestive of pericarditis. Furthermore, the differentiation of pericardium and pericardial fat can be difficult in post-contrast T1-weighted imaging. We use fat–water-separated Late-Enhancement-Imaging (mDIXON) to address this problem, although fat–water-swaps do occasionally occur. Visualization of the pericardium as a dark structure in the fat images facilitates differentiation. In our institution, we consider marked pericardial enhancement, or moderate pericardial enhancement in combination with either pericardial thickening or edema on T2 weighted imaging, as suggestive of acute pericarditis. While CMR studies in non-COVID chest-pain patients implicate an under-diagnosis of pericarditis using clinical criteria, the implications of a CMR-diagnosed pericarditis that lacks the clinical criteria for pericarditis are unknown [47].
For myocarditis post COVID-19-vaccination, the available epidemiological studies did not systematically incorporate CMR into the diagnostic workflow [27, 38]. CMR data is limited to small case series, possibly due to different referral patterns and public perception [28, 29, 48]. Reports of frequent myocarditis post COVID-19 in mid to late 2020 generated high awareness and lead to frequent referrals for suspected myocarditis even in cases of moderate to low pretest probability [21]. For myocarditis post vaccination, evidence was only beginning to emerge during our study period and referral was mostly confined to cases with strong clinical suspicion, reflected by the high rate of troponin positives in post-vaccination myocarditis case series, including our report (Table 5). Screening of asymptomatic vaccinated persons was not performed after vaccination as has happened after COVID-19 in athletes. In these screening studies after COVID-19, about ¾ of myocarditis cases were asymptomatic and would not have been discovered on clinical suspicion alone. The corresponding number of asymptomatic and oligosymptomatic myocarditis cases after COVID-vaccination is therefore yet unknown, hampering comparisons of incidence rates.
A recent epidemiological study comparing rates of myocarditis after COVID-19 and after vaccination using matched data from the English national health system and the English national immunization database found a 4 to 40 fold higher incidence of myocarditis in the 28 days following COVID-19 compared to 28 days following COVID-19 vaccination [49]. A recent meta-analysis of 11 studies found an incidence of 18.2 perimyocarditis cases per million COVID-19-vaccine doses, lower than that of other vaccines [50]. This would translate to an estimated 82 cases in Berlin in the study period, which seems consistent with our data, given the high hospital density in Berlin.
Cardiac findings other than myocarditis were more common in our post-COVID-19-cohort compared to the post-vaccination-cohort. Cardiac complications after respiratory infections other than COVID are common. The incidence of myocardial infarction rises after pneumonia and vaccination against influenza reduces cardiovascular mortality [51, 52]. The link between influenza and myocarditis is less clear and CMR-studies are missing [53]. Myocarditis after influenza vaccination is exceedingly rare [54].
Evolving diagnostic criteria for myocarditis
The diagnosis of myocarditis can be challenging due to its heterogeneous presentation and evolving diagnostic criteria [55]. A definite diagnosis requires histological confirmation via endomyocardial biopsy or post-mortem examination [55]. The 2013 position statement by the European society of cardiology (ESC) suggests a diagnosis of clinically suspected myocarditis in the presence of clinical symptoms and at least one of four clinical criteria, one of which is evidence of LGE and/or edema on CMR [56]. In asymptomatic cases, at least two clinical criteria are required. While CMR has good diagnostic accuracy in “Infarct-like” myocarditis, presenting with fever, chest pain, cardiac enzyme elevations, and ST-segment elevations on ECG, its accuracy is markedly reduced in cardiomyopathic and arrhythmic presentations [57]. The CMR criteria for myocarditis are evolving as well, with the removal of “early gadolinium enhancement” and the introduction of mapping techniques in the updated LCC 2018. These mapping-techniques are also still evolving, with site-dependent normal values and an incomplete understanding of confounders such as sex, age, comorbidities and artifacts [58, 59]. CMR-based strain measurements such as feature tracking and SENC show promise as additional diagnostic and prognostic parameters in myocarditis but have yet to be incorporated into official recommendations. (33454266, 32682718) In summary, these factors contribute to a fragmentation of diagnostic approaches: Some groups did not use CMR at all. Some groups required both updated Lake Louise Criteria, others just one. Some groups required matching LGE and edema and some excluded LGE on the RV-insertion-site. This, in combination with referral bias, mostly solves the conundrum of widely varying myocarditis rates between centers. To demonstrate this point and facilitate comparison of our results with other studies, we reassessed our myocarditis cases based on different diagnostic criteria (Table 7).
Limitations
Due to infectiosity the post-COVID-19-patients were not examined during the acute stage of the disease but at a mean of 112 ± 76 days after the first positive PCR test. Therefore, a myocarditis at the beginning of the COVID-19 disease might no longer show edema at the time of CMR. Myocardial damage as assessed by LGE on the other hand is usually permanent and would still be visible at the time of CMR as a sign of subsided myocarditis. We found only three cases of possible subsided myocarditis compared to nine cases with edema still present in the post-COVID-19 cohort, which indicates that most cases of myocarditis were still in their acute or subacute phase. In the post-vaccination-cohort, time from vaccination to CMR was rather long as well with 44 ± 35 days. We attribute this to the low awareness of myocarditis as a possible complication of vaccination during the examination period and the inclusion of patients with unremarkable basic cardiac workup but intractable symptoms, who underwent a period of watchful waiting.
Examinations were performed on a 3T and a 1.5T scanner. Because T1 and T2 relaxation times differ between field strengths, comparisons of those can only be drawn within the same field strength.
Our patients represent an unselected cohort of clinical all-comers, therefore confounders in patient selection might be present. As our clinic sees only a part of myocarditis-patients in Berlin, our numbers do not and cannot reflect the true prevalence of myocarditis both post COVID-19 and post vaccination. Our findings suggest a different referral pattern between both groups at the time, with a lower threshold for CMR in post-COVID-19-patients compared to post-vaccination-patients. Patients after vaccination presented with higher cardiac enzymes and had more evident clinical symptoms at the time of the examination.
Conclusion
Despite the manifold higher number of vaccinations than COVID-19-infections, more patients after COVID-19 than after vaccination presented to our clinic with suspected cardiac involvement. Of those, CMR offered a diagnosis with therapeutic relevance in 32.7% of patients after COVID-19 and 81.5% of patients after vaccination. Applying stricter CMR criteria for myocarditis reduced cases post-COVID-19 but not post vaccination, reflecting more atypical myocarditis presentations post-COVID-19. Further epidemiological studies are needed to refine diagnostic criteria and determine the true prevalence and type of heart damage related to both COVID-19-disease and COVID-19-vaccines.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Doeblin P, Kelle S (2021) Gong after COVID-19-myocarditis. Eur Heart J Cardiovasc Imaging (in press)
Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W et al (2020) Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol 311:116–121
Halushka MK, Vander Heide RS (2021) Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol 50:107300
Tschope C, Sherif M, Anker MS, Geisel D, Kuehne T, Kelle S (2021) COVID-19-convalescence phase unmasks a silent myocardial infarction due to coronary plaque rupture. ESC Heart Fail 8(2):971–973
Carfi A, Bernabei R, Landi F (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–605
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397(10270):220–232
Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA et al (2021) Long COVID in a prospective cohort of home-isolated patients. Nat Med 27(9):1607–1613
Shah W, Hillman T, Playford ED, Hishmeh L (2021) Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 372:n136
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al (2009) Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 53(17):1475–1487
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U et al (2018) Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 72(24):3158–3176
Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G et al (2021) High prevalence of pericardial involvement in College Student Athletes recovering from COVID-19. JACC Cardiovasc Imaging 14(3):541–555
Chen BH, Shi NN, Wu CW, An DA, Shi YX, Wesemann LD et al (2021) Early cardiac involvement in patients with acute COVID-19 infection identified by multiparametric cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 22(8):844–851
Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA et al (2021) COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation 143(6):609–612
Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M et al (2021) Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol 6(9):1078–1087
Hendrickson BS, Stephens RE, Chang JV, Amburn JM, Pierotti LL, Johnson JL et al (2021) Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation 143(19):1926–1928
Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C et al (2020) Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging 13(11):2330–2339
Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS et al (2020) COVID-19: myocardial injury in survivors. Circulation 142(11):1120–1122
Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G et al (2021) Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 42(19):1866–1878
Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G et al (2021) Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol 6(7):745–752
Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR et al (2021) SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation 144(4):256–266
Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J et al (2020) Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 5(11):1265–1273
Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP et al (2021) Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 6(1):116–118
Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE et al (2021) Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol 6(8):945–950
Vago H, Szabo L, Dohy Z, Merkely B (2020) Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. JACC Cardiovasc Imaging 14(6):1279–1281
Weckbach LT, Curta A, Bieber S, Kraechan A, Brado J, Hellmuth JC et al (2021) Myocardial inflammation and dysfunction in COVID-19-associated myocardial injury. Circ Cardiovasc Imaging 14(1):e012220
Seidel F, Kuehne T, Kelle S, Doeblin P, Zieschang V, Tschoepe C et al (2021) Cardiovascular magnetic resonance findings in non-hospitalized paediatric patients after recovery from COVID-19. ESC Heart Fail 8(6):5583–5588
Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L et al (2021) Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 6(10):1202–1206
Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN et al (2021) Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol 6(10):1196–1201
Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS et al (2021) Symptomatic acute myocarditis in 7 adolescents after pfizer-BioNTech COVID-19 vaccination. Pediatrics 148(3):e2021052478
Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D et al (2021) COVID-19 vaccine safety in adolescents aged 12–17 years—United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep 70(31):1053–1058
Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y et al (2021) Myocarditis after Covid-19 vaccination in a large Health Care Organization. N Engl J Med 385(23):2132–2139
Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR et al (2022) Myocarditis cases reported after mRNA-Based COVID-19 vaccination in the US From December 2020 to August 2021. JAMA 327(4):331–340
Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, Wald RM, et al (2022) Myocardial injury pattern at MRI in COVID-19 vaccine-associated myocarditis. Radiology 212559
Jahnke C, Doeblin P, Tanacli R, Witt U, Schneider M, Stehning C et al (2022) Case series of potential cardiac inflammation associated with various SARS-CoV-2 vaccinations assessed by cardiac MRI. Front Cardiovasc Med 9:829392
Schauer J, Buddhe S, Gulhane A, Sagiv E, Studer M, Colyer J, et al (2022) Persistent cardiac MRI findings in a cohort of adolescents with post COVID-19 mRNA vaccine myopericarditis. J Pediatr
Amir G, Rotstein A, Razon Y, Beyersdorf GB, Barak-Corren Y, Godfrey ME, et al (2022) CMR imaging 6 months after myocarditis associated with the BNT162b2 mRNA COVID-19 vaccine. Pediatr Cardiol
Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R et al (2021) Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 385(12):1078–1090
Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME et al (2021) Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep 70(27):977–982
Tanacli R, Doeblin P, Gotze C, Zieschang V, Faragli A, Stehning C et al (2021) COVID-19 vs. classical myocarditis associated myocardial injury evaluated by cardiac magnetic resonance and endomyocardial biopsy. Front Cardiovasc Med 8:737257
Kelle S, Bucciarelli-Ducci C, Judd RM, Kwong RY, Simonetti O, Plein S et al (2020) Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magn Reson 22(1):61
Arai AE, Schulz-Menger J, Berman D, Mahrholdt H, Han Y, Bandettini WP et al (2020) Gadobutrol-enhanced cardiac magnetic resonance imaging for detection of coronary artery disease. J Am Coll Cardiol 76(13):1536–1547
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update. J Cardiovasc Magn Reson 22(1):19
Robert Koch-Institut. COVID-19 Impfungen in Deutschland. https://raw.githubusercontent.com/robert-koch-institut/COVID-19-Impfungen_in_Deutschland/master/Aktuell_Deutschland_Bundeslaender_COVID-19-Impfungen.csv
LaGeSo Berlin. COVID-19 in Berlin, Fallzahlen und Indikatoren—Gesamtübersicht. https://www.berlin.de/lageso/gesundheit/infektionskrankheiten/corona/tabelle-indikatoren-gesamtuebersicht/index.php/index/all.csv?q=
Cosyns B, Plein S, Nihoyanopoulos P, Smiseth O, Achenbach S, Andrade MJ et al (2015) European Association of Cardiovascular Imaging (EACVI) position paper: multimodality imaging in pericardial disease. Eur Heart J Cardiovasc Imaging 16(1):12–31
Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J et al (2015) 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 36(42):2921–2964
Boniface N, Kley J, Lisko J, Mikolich B, Mikolich JR (2014) Abstract 12863: non-cardiac chest pain: is it really? Circulation 130(Suppl 2)
Hudson B, Mantooth R, DeLaney M (2021) Myocarditis and pericarditis after vaccination for COVID-19. J Am Coll Emerg Physicians Open 2(4):e12498
Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M et al (2022) Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 28(2):410–422
Lang RR, Kollengode R, Tan FL, Tai BC, Somani J, Fisher D, et al (2022) Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med
Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC et al (2015) Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 313(3):264–274
Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A et al (2013) Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 310(16):1711–1720
Baral N, Adhikari P, Adhikari G, Karki S (2020) Influenza myocarditis: a literature review. Cureus 12(12):e12007
Nagano N, Yano T, Fujita Y, Koyama M, Hasegawa R, Nakata J et al (2020) Hemodynamic collapse after influenza vaccination: a vaccine-induced fulminant myocarditis? Can J Cardiol 36(9):1554
Tschope C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB et al (2021) Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 18(3):169–193
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB et al (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34(33):2636–2648
Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R et al (2014) CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging 7(3):254–263
Doeblin P, Hashemi D, Tanacli R, Lapinskas T, Gebker R, Stehning C et al (2019) CMR tissue characterization in patients with HFmrEF. J Clin Med 8(11):1877
Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 19(1):75
Funding
Open Access funding enabled and organized by Projekt DEAL. SK was supported by an unrestricted research grant from Philips Healthcare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by PD, SA and CG. The first draft of the manuscript was written by PD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
PD owns stock of Siemens and Bayer. AF is a shareholder of BOCAhealthcare GmbH. CS is an employer of Philips Healthcare. BP reported receiving personal fees from Bayer, Bristol Myers Squib, Daiichi Sankyo, Medscape, MSD, Novartis, Stealth Peptides, and Vifor Pharma, and grants and personal fees from Astra-Zeneca. CT, BP and SK received funding from the DZHK (German Centre for Cardiovascular Research) and by the BMBF (German Ministry of Education and Research) and personal fees from Servier, outside of the current work. SK received an unrestricted research grant from Philips Healthcare and received lecture honoraria from Medis, NL.
Ethical approval and consent to participate
The study complies with the declaration of Helsinki and was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA2/020/21) as a retrospective analysis with a waiver of consent.
Consent for publication
Consent for publication has been obtained from all patients with individual data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doeblin, P., Jahnke, C., Schneider, M. et al. CMR findings after COVID-19 and after COVID-19-vaccination—same but different?. Int J Cardiovasc Imaging 38, 2057–2071 (2022). https://doi.org/10.1007/s10554-022-02623-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02623-x