Abstract
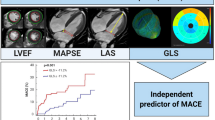
Global longitudinal strain (GLS) is a more sensitive prognostic factor than left ventricular ejection fraction (LVEF) in various cardiac diseases. Little is known about the clinical impact of GLS changes after acute myocardial infarction (AMI). The present study aimed to explore if non-improvement of GLS after 3 months was associated with higher risk of subsequent composite cardiovascular events (CCVE). Patients with AMI were consecutively included at a secondary care center in Norway between April 2016 and July 2018 within 4 days following percutaneous coronary intervention. Echocardiography was performed at baseline and after 3 months. Patients were categorized with non-improvement (0 to − 100%) or improvement (0 to 100%) in GLS relative to the baseline value. Among 214 patients with mean age 65 (± 10) years and mean LVEF 50% (± 8) at baseline, 50 (23%) had non-improvement (GLS: − 16.0% (± 3.7) to − 14.2% (± 3.6)) and 164 (77%) had improvement (GLS: − 14.0% (± 3.0) to − 16.9% (± 3.0%)). During a mean follow-up of 3.3 years (95% CI 3.2 to 3.4) 77 CCVE occurred in 52 patients. In adjusted Cox regression analyses, baseline GLS was associated with all recurrent CCVE (HR 1.1, 95% CI 1.0 to 1.2, p < 0.001) whereas non-improvement versus improvement over 3 months follow-up was not. Baseline GLS was significantly associated with the number of CCVE in revascularized AMI patients whereas non-improvement of GLS after 3 months was not. Further large-scale studies are needed before repeated GLS measurements may be recommended in clinical practice.
Trial registration: Current Research information system in Norway (CRISTIN). Id: 506563
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of patients who have been treated with early percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) are discharged without reduced left ventricular ejection fraction (LVEF) [1,2,3]. Reduced global longitudinal strain (GLS) is a more sensitive predictor for cardiac events and remodeling than LVEF post-AMI, especially among patients without reduced LVEF [3,4,5,6]. The question arises whether improvement of GLS following AMI is associated with favorable clinical outcomes, such as recently reported for improvement of LVEF [7]. In a study of patients treated with PCI for ST-segment elevation myocardial infarction (STEMI), Antoni et al. [8] reported significant improvement of GLS at 3 months and between 3 and 12 months. Left anterior descending artery as culprit vessel, peak cardiac troponin T level and diastolic function were the independent predictors for improvement. Baron et al. [9] studied post AMI patients of whom 47% had STEMI and 90% had been treated with PCI. GLS significantly improved during the following 12 months, with independent predictors being initial impairment of LV function, including assessment with GLS, male gender, non-smoking and treatment with beta-blockers. None of these studies, however, explored the association between the improvement or non-improvement of GLS and subsequent composite cardiovascular events (CCVE).
In the present study, we aimed to explore the association between GLS improvement or non-improvement at baseline and 3 months following PCI treated AMI and clinical outcome during more than 3 years follow-up. Our hypothesis was that non-improvement of GLS predicted a higher incidence of CCVE than improvement.
Methods
Design and study population
A prospective, observational follow-up study of patients recruited from Vestfold Hospital Trust, a non-invasive, secondary care general hospital. Since 2005, all eligible patients with AMI have been transferred for invasive revascularization therapy at Oslo University Hospital (tertiary center). Following PCI, most patients return within 1–2 days for subsequent management at Vestfold. Stabilized patients were included consecutively within 4 days following PCI from April 1st 2016–July 31nd 2018 according to the following criteria:
-
The diagnosis of AMI type 1 [10] and PCI performed according to prevailing guidelines [11] at the time when the study was planned (2015).
-
Providing written consent for study participation.
Exclusion criteria
Baseline
-
Hemodynamically unstable patients (ongoing arrhythmia, heart failure, significant comorbidities) according to the opinion of the principal investigators.
-
Atrial fibrillation / flutter / irregular heart rhythm
-
Poor echocardiographic images according to the principal investigator`s opinion.
3 months
-
Not willing to attend the second examination
-
Recurrent AMI, development of heart failure requiring hospitalization or arrhythmias between the two echocardiographic studies.
-
Anticipated inability to attend the 3 months examination and life expectancy < 2 years
The study was approved by the Regional Ethics Committee of Health Region South-East, Norway (2015/2359).
Echocardiography
All echocardiographic examinations were performed with a General Electric scanner Vivid E9 (Vingmed Ultrasound, Horten, Norway) within 4 days of intervention. Whenever feasible, a new echocardiographic examination was performed after 3 months. In order to optimize the quality of recordings and minimize the influence of interobserver variability only two experienced operators (VR and JEO) performed the echocardiographic examinations.
Global longitudinal strain
Three to four consecutive heartbeats were recorded from each of the three apical views. End of systole was defined as aortic valve closure registered by continuous Doppler. Manual editing of the region of interest was performed, whenever found appropriate by the investigator, and in accordance with the recent recommendations [12]. As part of quality insurance all baseline and 3 months GLS values were reanalyzed blindly, and in case of a deviation of > 10%, a reassessment was performed of the registered value based upon consensus between the two echocardiographers involved in the study. We excluded images with suboptimal tracking of the endocardium in more than one segment in one single view, or if frame rate was below 50 Hz.
Improvement was defined as a relative decrease of GLS (i.e. to a more negative value) in percentage of the baseline value from ÷ 0% to ÷ 100%; and non-improvement as a relative increase (i.e. to a less negative value) from 0 to 100%. Patients with identical GLS on both occasions were categorized as improvers.
Conventional echocardiography
LV mass index was evaluated by M-mode in parasternal long axis. LVEF was measured by the biplane Simpson method. Maximal left atrial volume index (LAVI) was measured by the biplane area-length method. Pulsed and tissue Doppler were applied for E/e’ ratio using the average of e’ septal and e’ lateral velocities.
Follow up
Patients were followed until death or 31st October 2020 by telephone interviews and careful screening of all available hospital records. All patients were offered participation in our multidisciplinary cardiac rehabilitation program [13] and medical treatment was given according to present guidelines. The following CCVE were defined from the 3 months follow-up echocardiogram to the end-of follow-up, as judged from all available hospital records by two independent investigators who were blinded to the echocardiographic findings:
-
1.
Death
-
2.
Reinfarctions according to the universal definition [10]
-
3.
Hospitalization for heart failure
-
4.
Hospitalization for angina pectoris with a new coronary angiogram confirming progression of coronary artery stenoses requiring urgent PCI, or if not due to poor periphery of stenotic coronary arteries
-
5.
Hospitalization for ventricular arrhythmia
-
6.
New diagnosis of atrial fibrillation (AF) during follow-up if it was documented in a 12-channel ECG provided not being present before the index AMI
-
7.
Hospitalization for stroke / transitory ischemic attack
Statistical analysis
Data is either presented as mean ± SD or median and interquartile range (IQR) as appropriate. Normality was tested with the Shapiro–Wilk test, whenever visual assessment of the distribution was dubious. Differences of variables at baseline between the two groups according to change in GLS (from baseline to 3 months) were assessed with analysis of variance and Kruskal–Wallis for normal and non-normal distributions respectively. A chi square test or Fisher’s exact test was used for differences in categorical parameters. Survival analysis was performed to compare time from baseline to CCVE in the two groups, and identify covariates associated with the length of this time-interval. The time-scale was observation time, from operation to CCVE or stop of follow-up, which ever came first. Right censoring at end of follow-up occurred for those without CCVE. A Kaplan–Meier plot was used to compare time to CCVE between the two groups visually with corresponding log-rank test. Cox proportional hazards regression was performed to estimate relative incidence rates (hazard ratios) of CCVE and adjusted for relevant baseline covariates. In addition to assess the association between improvement of GLS and clinical outcome we introduced subsequent events as outcome in order to obtain higher precision. Tests for the assumption of proportional hazards were performed. We chose 2 models, testing both the hazard rates for first and for all (first + subsequent events) CCVE.
Model 1: A simplified approach including age decades, gender, baseline GLS and the two delta GLS categories using patients with non-improvement as reference (HR = 1).
Model 2: An extension with addition of the following co—variables (baseline): Previous AMI, STEMI with anterior wall location and baseline LVEF, also with the non-improvement group as reference.
For those with subsequent events, a sandwich variance estimator was used to account for dependence. The “survival” R-package was used for analysis [14]. All other statistical analysis were performed using SPSS version 25 (SPSS, Inc, Chicago, IL).
Reproducibility of repeated GLS measurements
Intra- and interobserver variability of GLS in our laboratory was tested in a previous study in 20 randomly selected patients. For the interobserver analyses, two observers investigated the same cine-loops blinded to the results of the other. For intra-observer analyses, one observer investigated the same cine-loops approximately 4 weeks apart. Intraclass correlation coefficient was 0.91 (0.77–0.97) for intraobserver variability and 0.84 (0.57–0.94) for interobserver variability.
Results
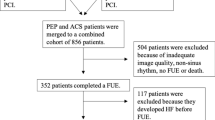
In all, 289 patients were screened for participation and reasons for exclusion in 53 patients are provided in Fig. 1. Of the remaining 236 patients, one patient (0.4%) was excluded due to hospitalization for heart failure, four (1.7%) due to recurrent AMI during the first 3 months and 17 (7.2%) did not wish to attend. Thus, 214 patients were eligible for the final study. There were no significant differences in clinical and echocardiographic characteristics between the total population included (n = 236) and the final study group (n = 214) at baseline (Supplemental file 1 and 2). Mean age in the study group was 65 (± 10) years, 25% were females, mean LVEF was 50% (± 8), 22 patients (10.3%) had LVEF < 40% and 17% had a previous MI. The percentage of STEMI was 53%, all treated with primary PCI at a median of 4.0 (IQR 3.0) hours after debut of symptoms. NSTEMI patients were treated with PCI after a median of 48 (IQR 37) hours. All patients were examined with baseline echocardiography after a median of 2 (IQR 1) days following PCI.
After 3 months follow-up, mean GLS had improved from − 14.4% (± 3.4) to − 16.2% (± 3.4), p < 0.001) i.e., a relative mean improvement of 12.5% of the baseline value. At that time GLS was not improved in 50 (23%) and improved in 164 patients (77%), of whom five had identical GLS on both occasions. Baseline characteristics are provided in Table 1. Patients with non-improvement had a higher number of stents implanted (p < 0.01) than improvers. In the subgroups with STEMI those with non-improvement had a longer mean symptom to revascularization time than improvers (p < 0.001). There were no significant difference between the number of patients where time to primary PCI was ≥ 10 h between improvers and non-improvers presenting with STEMI (p = 0.53).
Conventional echocardiographic data from the two groups are shown in Table 2, revealing no significant intergroup differences. Individual values for GLS at baseline and 3 months are presented in Fig. 2. The greatest improvement tended to occur among patients with the worst LV function. An opposite trend was noted among non-improvers. The correlation between baseline and delta GLS (change after 3 months) was strong (rho = 0.48, supplemental file 3). Bull’s eye plots from one patient with improvement and one with non-improvement at baseline and at 3 months follow-up are shown in Fig. 3.
Illustration of a patient with non-improvement and a patient with improvement of GLS 3 months from baseline. a Bull’s eye plot from a patient with non-improvement of GLS from − 13.8% (A1) at baseline to − 10.1% (A2) after 3 months. Coronary angiography 7 days after symptom onset revealed native right coronary artery and a bypass to right posterior descending artery from previous coronary artery bypass surgery as culprit of a new non-ST-segment elevation myocardial infarction. Both lesions were treated with percutaneous coronary intervention. Maximum troponin T level was 116 ng/L. b Bull’s eye plot from a patient with improvement of GLS from − 14.9% (B1) at baseline to − 17.3% (B2) at 3 months. Coronary angiography 2 days after debut of symptoms found an occluded circumflex artery, which was treated with percutaneous coronary intervention. No other significant coronary artery stenosis were found. Maximum troponin T level was 3553 ng/L
During a mean follow-up period of 3.3 years (95% CI 3.2–3.4), 77 CCVE occurred in 52 patients (Table 3). Of the 23 angina events, all with angiographically verified progression, 18 were treated with urgent PCI and the remaining five had poor peripheries. The majority of recurrent AMI was NSTEMI (89%), and urgent revascularization was performed in 9/18 of these events (8 PCI and 1 CABG). All 12 AF cases were subjected to treatment with a novel anticoagulant and predominantly rate control. None had cardioversion at the time of new-onset AF diagnosis. A Kaplan–Meier plot reflecting time to first CCVE among patients with non-improvement and improvement are shown in Fig. 4.
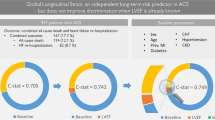
Model 1 of the Cox regression analysis is presented in Table 4. For first CCVE, using the age group 40–50 years as reference, only belonging to the 50–60 years decade (p < 0.05) was associated with lower incidence of CCVE. For all CCVE, patients in age decades 50–60 (p < 0.01) and 60–70 (p < 0.05) had a lower incidence than those in the age decade 40–50 years. In addition, baseline GLS but not GLS changes after 3 months was significantly associated with the number of CCVE (p < 0.05).
In the more comprehensive Model 2 (Table 5), belonging to the age decade 50–60 years was again associated with a lower incidence of first CCVE than those aged 40–50 years (p < 0.01). Previous AMI had a significant relationship with a higher incidence of first CCVE (p < 0.05). For all CCVE, the same pattern was found where patients in the decade 50–60 (p < 0.01), but also 60–70 years (p < 0.05) had a significantly lower incidence than those aged 40–50 years, and a history of previous AMI was a significant predictor of increased CCVE (p < 0.05). In this model neither baseline GLS nor GLS changes after 3 months were significantly associated with the incidence of subsequent CCVE.
Discussion
In the present study baseline GLS, but not GLS changes after 3 months, was significantly associated with the number of CCVE. To our knowledge, this is the first study to assess the prognostic importance of non-improved vs. improved GLS after 3 months in a post AMI population with the majority of patients having preserved LVEF.
No patients have been lost to follow-up after 3 months, and adequate hospital records have been available in this prospective, single-center study. In general, baseline differences in a comprehensive dataset of clinical and echocardiographic variables were small. Patients with non-improvement needed more stents and had a slightly lower LVEF at baseline than improvers. Although improvers with STEMI appeared to have a shorter symptom-to-needle time, the number of patients with delays above 10 h was similar in the two groups. The study has incorporated detailed information of patient management both at baseline and after 3 months. No major intergroup differences were found for medical treatment or participation cardiac rehabilitation. We chose to exclude a minority of patients due to cardiac events before the second examination since events could have had important influence on LV function at 3 months. This design, however, has a trend for selection of low-risk post-AMI patients. We believe that our findings are valid for the population studied, although obviously limited to the occurrence of coronary events and not for development of more serious endpoints such as heart failure or fatalities. Another matter to clarify is the possible influence of a regression to the mean phenomenon versus the influence of true biological changes of GLS after 3 months. Clearly, the changes cannot be explained by regression to the mean alone.
GLS improvement after 3 months can possibly be explained by myocardial stunning which is a reversible post-ischemic dysfunction of the myocardium that recovers during weeks to months [8, 15]. Patients with improvement had generally the worst LV function by GLS at baseline. Those with the poorest values may have had the highest potential for improvement. On the opposite, patients with non-improvement started with better LV function by GLS and turned out to have worse LV function than improvers at 3 months. Therefore, it may well be that the deterioration of LV function seen among patients with relatively good GLS values at baseline have resulted in a clinical course well comparable with improvers with poorer GLS values at baseline. The role of baseline GLS as predictor of CCVE was confirmed for all CCVE in the simple Model 1, but was not significant in the more comprehensive Model 2. Model 2 comprised several important confounders such as baseline LVEF, anterior wall STEMI and, especially, previous AMI, which may have influenced the prognostic role of baseline GLS, as opposed to the findings in most other studies. To us, this observation was surprising and may also be the result of a relatively small numbers of patients and events. On the other hand, most previous studies have evaluated the prognostic role of GLS on the occurrence of more serious endpoints such as fatalities and heart failure [4, 5], and not predominantly coronary events such has angina and recurrent AMI as in our study.
So far, studies exploring the association between echocardiographic assessment of changes in systolic function over time and outcome have mostly incorporated conventional echocardiographic measurements [7, 16]. The superior prognostic role of baseline GLS compared to LVEF has been verified in several larger studies incorporating the incidence of a broader selection of clinical endpoints such as higher numbers of mortality, ventricular arrhythmias, heart failure and reinfarctions [3, 7, 17,18,19,20,21]. Neither of these studies, however, had included GLS changes as a co-variable in adjusted analyses.
Only a few studies have followed the time course of GLS in AMI patients. Antoni et al. [8] arbitrarily defined improvement as ≥ 10% relative increase of baseline GLS and overall mean GLS improved by 17%, with 54% of their patients being categorized as improvers after 3 months. In the study of Baron et al. [9] a 10% relative GLS improvement was reported after 1 year, and improvement in GLS was associated 3 months with impairment of LV function (by LVEF, WMSI or GLS) at baseline. A Polish study by Wdoviak-Okrojec et al. [22] studied the improvement of regional systolic longitudinal strain on days 1,2,3,7, 30 and 180 days following AMI treated with PCI. The largest improvement occurred between days 1 and 2, with non-significant improvement thereafter. Neither of these studies reported the incidence of non-fatal cardiac events that might have influenced GLS changes during follow-up. In context of the findings in the Polish study [22], it is of importance that our baseline GLS has been derived at the time when the most pronounced improvement may have had taken place. In spite of this, we still observed an increase at a later time point, as also observed in the two aforementioned studies [8, 9].
An interesting finding was the non-linear association of CCVE with age. The youngest patients (i.e. 40–50 year) seemed to have a significantly higher risk of recurrent CCVE than the middle-aged (i.e. 50–60 and, in part 60–70 years). A possible explanation might be a more severe disease among those who sustain AMI at a younger age, or higher prevalence of unhealthy lifestyle behavior, as recently documented in a representative Norwegian post-AMI study [23]. Clearly, larger prospectively conducted long-term follow-up studies are needed to further explore the younger subgroup and eventually detect an “age paradox”.
One important question is whether a repeated echocardiographic examination including GLS measurements is necessary for prognostic purposes after 3 months or more. Apparently, from the results of our multi-adjusted analyses, this would seem unnecessary. On the other hand, in patients with poor baseline GLS the finding of major improvement at 3 months will render valuable prognostic importance for both the patient and treating physician. With our limited number of patients and events in mind, larger studies are needed before recommending such controls in a broader scale.
The major limitation of this study is the relatively small number of patients and a low number of events. In order to optimize the strength of our findings we included both first and subsequent events to represent all events. In addition, we added new onset AF among CCVE, which by many would be regarded as a minor event. This was, however, based upon our own experience from the ACTION study [24], where new-onset AF with the same diagnostic criteria as in the present study was a significant harbinger of death and heart failure among patients with stable CHD and LVEF ≥ 40%. Of note in this context is that 90% of our patients had LVEF ≥ 40% at baseline. This prognostic role of AF in patients with CHD was further corroborated in a recent review [25]. Since AF represented only 15% of all events, the inclusion of AF is not considered to have any major impact on our findings. Although an exploration of degrees of improvement and non-improvement associated with the incidence of CCVE would have been desirable, we deliberately avoided a subdivision in several subgroups. In this study, such a subdivision would be hampered by type 2 errors.
Conclusion
Baseline GLS was significantly associated with the number of CCVE in revascularized AMI patients whereas non-improvement in GLS over 3 months follow-up was not. Although these findings do not support a routine practice of repeated GLS measurements, further large-scale studies are needed to answer this question.
Data availability
Data are available upon request.
References
Hambraeus K, Held C, Johansson P, Cider Å, James S, Lagerqvist B et al (2014) SWEDEHEART annual report 2012. Scand Cardiovasc J 48:2–133
Ruddox V, Otterstad JE, Atar D, Bendz B, Edvardsen T (2018) In current clinical practice, after percutaneous coronary intervention for acute myocardial infarction, are β-blockers prescribed for heart failure or as secondary prevention? A pilot study. Cardiology 140:152–154
Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ et al (2013) Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 61:2365–2373
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100:1673–1680
Joyce E, Hoogslag GE, Leong DP, Debonnaire P, Katsanos S, Boden H et al (2014) Association between left ventricular global longitudinal strain and adverse left ventricular dilatation after ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging 7:74–81
Potter E, Marwick TH (2018) Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging 11:260–274
Chew DS, Heikki H, Schmidt G, Kavanagh KM, Dommasch M, Bloch Thomsen PE et al (2018) Change in left ventricular ejection fraction following first myocardial infarction and outcome. JACC Clin Electrophysiol 4:672–682
Antoni ML, Mollema SA, Atary JZ, Borleffs CJW, Boersma E, van de Veire NRL et al (2010) Time course of global left ventricular strain after acute myocardial infarction. Eur Heart J 31:2006–2013
Baron T, Christersson C, Hjortén G, Hedin EM, Flachskampf FA (2018) Changes in global longitudinal strain and left ventricular ejection fraction during the first year after myocardial infarction: results from a large consecutive cohort. Eur Heart J Cardiovasc Imaging 19:1165–1173
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD et al (2012) Third Universal definition of myocardial infarction. Eur Heart J 33:2551–2567
Windecker S, Kolh P, Alonso F, Collet JP, Cremer J, Falk V et al (2014) 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 35:2541–2619
Negishi K, Negishi N, Kurosawa K, Hristova K, Popescu BA, Vinereanu D et al (2015) Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc Imaging 8:489–492
Peersen K, Munkhaugen J, Gullestad L, Liodden T, Moum T, Dammen T et al (2017) The role of cardiac rehabilitation in secondary prevention after coronary events. Eur J Prev Cardiol 24:1360–1368
Therneau T (2020). A package for survival analysis in R. R package version 3.1–12, http://CRAN.R-project.org/package=survival
Bolli R (1992) Myocardial, “stunning” in man. Circulation 86:1671–1691
Otterstad JE, St. John Sutton MG, Frøland G, Holme I, Skjærpe T, Hall C (2002) Prognostic value of two-dimensional echocardiography and N-terminal proatrial natriuretic peptide following an acute myocardial infarction. Assessment of baseline values (2–7 days) and changes at 3 months in patients with a preserved systolic function. Eur Heart J 23:1011–1020
Sjøli B, Grenne B, Smiseth OA, Edvardsen T, Brunvand H (2011) The advantage of global strain compared to left ventricular ejection fraction to predict outcome after acute myocardial infarction. Echocardiography 28:556–563
Munk K, Andersen NH, Terkelsen CJ, Bibby BM, Johnsen SP, Bøtker HE et al (2012) Global left ventricular systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr 25:644–651
Haugaa K, Grenne BL, Eek CH, Ersbøll M, Valeur N, Svendsen JH et al (2013) Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging 6:841–850
Choi SW, Park J-H, Sun BJ, Park Y, Kim YJ, Lee IS et al (2015) Impaired two-dimensional global longitudinal strain of left ventricle predicts adverse long-term clinical outcomes in patients with acute myocardial infarction. Int J Cardiol 196:165–167
Stampehl MR, Mann DL, Ngyuen JS, Cota F, Colmenares C, Dokainish H (2015) Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular function. Echocardiography 32:71–78
Wdowaik-Okrojek K, Wejner-Mik P, Kasprzak J, Lipiec P (2019) Recovery of regional systolic and diastolic myocardial function after acute myocardial infarction evaluated by two-dimensional speckle tracking echocardiography. Clin Phys Funct Imaging 39:177–181
Sverre E, Peersen K, Husebye E, Gjertsen E, Gullestad L, Moum T et al (2017) Unfavourable risk factor control after coronary events in routine clinical practice. BMC Cardiovasc Disord 17:40–49
Otterstad JE, Kirwan B-A, Lubsen J, deBrouwer S, Fox KAA, Corell P, Poole-Wilson P (2006) Correlates and outcome of atrial fibrillation in stable symptomatic coronary disease. Scand Cardiovasc J 40(40):152–159
Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE (2017) Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol 24:1555–1566
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JEO, TE, JM, OK, ACML, VR, BB and IBN. The first draft of the manuscript was written by JEO and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Regional Ethics Committee of Health Region South-East, Norway (2015/2359).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otterstad, J.E., Norum, I.B., Ruddox, V. et al. Prognostic impact of non-improvement of global longitudinal strain in patients with revascularized acute myocardial infarction. Int J Cardiovasc Imaging 37, 3477–3487 (2021). https://doi.org/10.1007/s10554-021-02349-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02349-2