Abstract
High altitude (HA) exposure has been considered as a cardiac stress and might impair ventricular diastolic function. Atrial contraction is involved in ventricular passive filling, however the atrial performance to HA exposure is poorly understood. This study aimed to evaluate the effect of short-term HA exposure on bi-atrial function. Physiological and 2D-echocardiographic data were collected in 82 healthy men at sea level (SL, 400 m) and 4100 m after an ascent within 7 days. Atrial function was measured using volumetric and speckle-tracking analyses during reservoir, conduit and contractile phases of cardiac cycle. Following HA exposure, significant decreases of reservoir and conduit function indexes were observed in bi-atria, whereas decreases of contractile function indexes were observed in right atrium (RA), estimated via RA active emptying fraction (SL 41.7 ± 13.9% vs. HA 35.4 ± 12.2%, p = 0.001), strain during the contractile phase [SL 13.5 (11.4, 17.8) % vs. HA 12.3 (9.3, 15.9) %, p = 0.003], and peak strain rate during the contractile phase [SL − 1.76 (− 2.24, − 1.48) s−1 vs. HA − 1.57 (− 2.01, − 1.23) s−1, p = 0.002], but not in left atrium (LA). In conclusion, short-term HA exposure of healthy individuals impairs bi-atrial performance, mostly observed in RA. Especially, atrial contractile function decreases in RA rather than LA, which seems not to compensate for decreased ventricular filling after HA exposure. Our findings may provide a novel evidence for right-sided heart dysfunction to HA exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing number of lowlanders visit high altitude (HA) for work or leisure. However, HA exposure challenges cardiac function to meet the tissue metabolic demand for oxygen under hypoxic conditions [1]. It has been well established that the cardiac response to HA exposure presents preserved ventricular systolic function, impaired ventricular diastolic function, and elevated pulmonary arterial pressure [2]. Atrial contraction is the final component during ventricular diastole and contributes approximately 15% to 20% of stroke volume as a compensatory mechanism [3,4,5]. Evidence is lacking regarding atrial response to HA exposure. Until recently, Sareban et al. [6, 7] reported unchanged left atrial (LA) and enhanced right atrial (RA) contractile function after a few hours following an ascent to 4559 m. It actually takes several days to acclimate to HA conditions with cardiac output returning to normal through a higher heart rate and lower stroke volume [8]. Nevertheless, no studies to date have systematically described atrial performance under short-term HA exposure. HA exposure induces hypoxic pulmonary hypertension, and consequently increases right ventricular (RV) afterload, directly conducting to RA. Due to differences of pressure and resistance from vascular attachments, RA performance may be different from LA after HA exposure. Thus, it is of great value to evaluate the effect of short-term HA exposure on bi-atrial function, which can provide a novel insight into cardiac adaption to altitude exposure.
The atrium plays an important role in modulating ventricular filling by means of three phases: the reservoir phase during ventricular systole, the conduit phase during ventricular early diastole and the contractile phase during ventricular late diastole [9]. Doppler echocardiography has been previously used to assess relative atrial function, however it is subject to error because of angle dependence and non-specificity [10,11,12]. Recently, speckle-tracking echocardiography (STE) gradually supersedes Doppler imaging, which can quantify regional and global atrial myocardial deformation representing intrinsic myocardial properties [13, 14]. In this study, we aimed to investigate the effect of short-term HA exposure on bi-atrial function using STE and identify the related factors.
Methods
Study population and procedure
Healthy men from Han ethnicity aged 18–45 years old and permanently living below 500 m above sea level (asl) were recruited in June 2013. We excluded the subjects with the following: known cardiovascular and pulmonary disease (such as congenital heart disease, valvular disease, arrhythmia, chronic obstructive pulmonary disease, asthma), previous history of exposure to altitude above 2500 m asl in the past 6 months, and missing data or poor quality images. Finally, 82 subjects were enrolled in the analysis. The experimental protocol was registered under the Chinese Clinical Trial Registration (No: ChiCTR-RCS-12002232, http://www.chictr.org.cn). The study received approval by the Clinical Research Ethics Committee of the Third Military Medical University (Army Medical University) (NO: 2012015), in accordance with Declaration of Helsinki, and all subjects granted informed content for participation.
All subjects ascended to Litang (Sichuan, China, 4100 m asl) from Yanggongqiao (Chongqing, China, 400 m asl) by bus within 7 days. The subjects enrolled in our study underwent clinical examination and standard transthoracic echocardiography at sea level (SL, 400 m asl) and in 5 ± 2 h after arrival at 4100 m.
Clinical examination
Clinical data recorded for all subjects included age, height, and weight. Body mass index (BMI) and body surface area (BSA) were calculated according to the customary formula [15]. Blood pressure was measured by Omron HEM-6200 (Japan) after resting for at least 5 min. Arterial pulse oxygen saturation (SpO2) was measured using a pulse oximeter (Nonin ONYX OR9500, USA).
Echocardiographic image acquisition
The subjects underwent standard transthoracic echocardiography by an experienced cardiac sonographer. A commercially available CX50 ultrasound machine (Philips Ultrasound System, Andover, MA, USA) equipped with a 2.5 MHz frequency transducer was used to acquire images with a frame rate of 70–90 fps. The electrocardiogram connected to the ultrasound system recorded heart rate (HR) during the examination. All images were acquired in accordance with the recommendations of the American Society of Echocardiography [16]. The echocardiographic images were saved digitally and analyzed offline by two independent sonographer blinded to the data, using a commercially available workstation (QLAB version 10.5, Philips Healthcare, Andover, MA, USA).
Two-dimensional and Doppler echocardiography
Ventricular area and volume were measured during ventricular end-systole and end-diastole by two-dimensional (2D) echocardiography to calculate left ventricular ejection fraction (LVEF) [17] and RV fractional area change (FAC) [18]. Atrial volumes were obtained by tracing the atrial endocardium using Simpson’s method. Atrial maximal volume (Vmax) was obtained in end-systole at the onset of mitral/tricuspid valve opening, atrial minimal volume (Vmin) was obtained at the onset of mitral/tricuspid valve closure by QRS complex of ECG, and atrial pre-systolic volume (Vpre-A) was obtained preceding the P wave. All volume measurements were indexed to the BSA, and used to calculate atrial phasic emptying fractions, including total (EFtot), passive (EFpass) and active (EFact) components [10, 19].
From the pulsed-wave Doppler echocardiography of blood flow velocities at mitral and tricuspid valves, the peak early diastolic E-wave velocity, peak late diastolic A-wave velocity and peak tricuspid regurgitant (TR) velocity (TRV) were acquired. Systolic pulmonary arterial pressure (sPAP) was calculated as follows: 4 × TRV 2 + 5 mmHg (an estimated central venous pressure) [20, 21]. From pulsed-wave tissue Doppler images of mitral and tricuspid annuli, ventricular systolic S′ velocity, early diastolic E′ velocity, and late diastolic A′ velocity at the septal and lateral walls were measured.
Speckle-tracking echocardiography
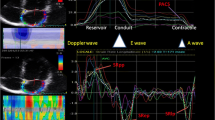
Atrial phasic strain and strain rate (SR) were obtained by 2D-STE. A 3-point click on the endocardial surface of the atrium and then the endocardial-epicardial borders were traced automatically by the system in four-chamber view. The optimized region of interest was manually adjusted for adequate speckle tracking. The software divided the region into seven segments, and generated strain and SR curves for each myocardial segment. The frame at QRS wave onset was used as the first reference frame. The atrial strain and peak SR during ventricular systole, early and late diastole were measured to evaluate atrial reservoir (Sr, pSRr), conduit (Scd, pSRcd), and contractile (Sct, pSRct) function respectively according to the recommendations of the European Society of Cardiology [22] as in Fig. 1. The noninvasive atrial stiffness index was calculated as the ratio of average E/E′ to Sr [23].
Two-dimensional speckle-tracking echocardiographic assessment of atrial function. Measurement of left atrial (A) and right atrial (B) reservoir, conduit and contractile function by strain and strain rate curves. LA left atrial, RA right atrial, Sr strain during the reservoir phase, Scd strain during the conduit phase, Sct strain during the contractile phase, pSRr peak strain rate during the reservoir phase, pSRcd peak strain rate during the conduit phase, pSRct peak strain rate during the contractile phase
Pulmonary function test
Pulmonary function test was conducted in 42 of these subjects who were randomly assigned. Spirometry was performed with a portable spirometer (Minato AS-507; Minato Medical Science Co., Ltd., Osaka, Japan) in compliance with standard techniques [24]. Pulmonary function measurements included forced vital capacity, forced expiratory volume in the first second, and maximum mid-expiratory flow. Subsequently, residual volume and total lung capacity were calculated.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 (Inc., La Jolla, USA). The normality of continuous variables was tested by the Kolmogorov–Smirnov test. Continuous variables with a normal distribution were expressed as the mean ± standard deviation (SD), Continuous variables with a non-normal distribution were expressed as the median (interquartile range), and categorical variables were expressed as the counts and proportions. Paired-t test or Wilcoxon matched-pairs signed rank test were used for the comparison of continuous variables, as appropriate. Pearson’s correlation was performed to analyze relationships among normally distributed continuous variables, and Spearman’s correlation was used for non-normally distributed statistics. A p value < 0.05 was defined as statistically significant.
Intra- and inter-observer variabilities were assessed in 20 randomly selected subjects. Inter-observer variability was performed by two independent observers, and intra-observer variability was performed by the same observer at least 1 month apart. Both the intra-observer and inter-observer variabilities were tested using the intra-class correlation coefficient (ICC) by Cronbach’s α.
Results
The effect of HA exposure on cardiac function
The mean age of the subjects was 20 (19–21) years old. The cardiac response to short-term HA exposure was presented in Table 1. Following HA exposure, increases in systolic blood pressure, diastolic blood pressure and HR, and decrease in SpO2 were observed. For LV parameters, unchanged EDV index and significantly decreased ESV index, thus, increased LVEF were observed. For RV parameters, significantly decreased EDA index while unchanged ESA index, and consequently decreased RV FAC were recorded at HA. Additionally, both mitral and tricuspid E/A decreased after HA exposure. As expected, the proportion of subjects with tricuspid regurgitation was greater at HA. Thus, sPAP significantly increased after HA exposure. From pulsed-wave Doppler echocardiography (Table 2), no significant change was observed in mitral and tricuspid S' and E'. However, decreased A' was observed at tricuspid annulus, but not at mitral annulus. Besides, E/E' decreased at both mitral and tricuspid valves.
The effect of HA exposure on bi-atrial phasic volumetric and strain parameters
The comparisons of atrial phasic function assessed by volume and speckle-tracking analysis between before and after HA exposure were presented in Table 3. Significant decreases were observed in the LAVmax and LAVpre-A, but not in RA volume indexes. However, a trend towards increase was observed in RAVmin after HA exposure. Besides, significant decreases were demonstrated in RAEFtot (SL 59.7 ± 11.6% vs. HA 54.5 ± 12.3%, p = 0.001), RA expansion index [SL 154.8 (97.5, 215.9) % vs. HA 117.3 (89.4, 171.7) %, p = 0.006], and RAEFact (SL 41.7 ± 13.9% vs. HA 35.4 ± 12.2%, p = 0.001) after ascending to HA, but not in LA indexes. Table 4 showed highly significant decreases in RA strain during reservoir (SL 43.5 ± 10.0% vs. HA 35.8 ± 9.5%, p < 0.001), conduit (SL 29.1 ± 7.5% vs. HA 23.3 ± 6.8%, p < 0.001) and contractile phases [SL 13.5 (11.4, 17.8)% vs. HA 12.3 (9.3, 15.9) %, p = 0.003], and LA strain during reservoir and conduit phases after HA exposure. Besides, for SR, RA decreased during reservoir [SL 1.98 (1.62, 2.47) s−1 vs. HA 1.70 (1.41, 2.09) s−1, p < 0.001], conduit [SL − 1.96 (− 2.37, − 1.59) s−1 vs. HA − 1.72 (− 2.14, − 1.42) s−1, p = 0.037], and contractile phases [SL − 1.76 (− 2.24, − 1.48) s−1 vs. HA − 1.57 (− 2.01, − 1.23) s−1, p = 0.002]. However, LA SR only decreased during reservoir phase (Fig. 2). Additionally, the bi-atrial stiffness indexes were not affected by HA exposure.
Comparisons of atrial phasic function between left and right atria under high altitude exposure. *p < 0.05; **p < 0.01. Abbreviations as in Fig. 1
Effect of SpO2 and TR on RA contractile function at HA
The subjects were stratified according to the decline of SpO2 after HA exposure. As presented in Fig. 3, significant decreases of RAEFact, RASct and pRASRct were observed in the group with higher decline of SpO2 (p < 0.05), but not in the lower group. Additionally, according to previous study that any increase in TR was associated with progressive increase in pressure of right-sided heart [25], the subjects were stratified according to the presence of TR at HA. As presented in Fig. 3, significant decreases of RAEFact, RASct and pRASRct were observed in subjects with TR (p < 0.05) after HA exposure, but not in subjects without TR.
Effect of oxygen saturation and tricuspid regurgitation on right atrial contractile function at high altitude. The subjects were stratified into two groups according to the decline of SpO2 (A, B and C) or the presence of TR after high altitude exposure (D, E and F), respectively. *p < 0.05; **p < 0.01. SpO2, arterial pulse oxygen saturation; TR tricuspid regurgitation. Other abbreviations as in Fig. 1
Correlations of RA phasic function with other parameters
The correlations of RA phasic function with physiological and other echocardiographic parameters after HA exposure were illustrated in Supplementary Table 1. ΔRAEFtot (r = 0.22, p = 0.047) showed a positive correlation with age. ΔRASr (r = − 0.30, p = 0.007), ΔRAScd (r = − 0.28, p = 0.011) and ΔpRASRr (r = − 0.31, p = 0.005) showed negative correlations with BMI. ΔRASr (r = − 0.26, p = 0.018) and ΔpRASRcd (r = 0.26, p = 0.020) showed significant correlations with ΔSpO2. ΔRAEFact (r = − 0.24, p = 0.032) showed negative correlations with Δtricuspid E/A. ΔRAEFtot (r = 0.32, p = 0.042) and ΔRAEFact (r = 0.39, p = 0.010) showed significantly positive correlations with ΔsPAP. Additionally, the correlations of RA contractile function with pulmonary function after HA exposure were presented in Supplementary Table 2. However, no significant correlations of RAEFact, RASct and pRASRct with any pulmonary function indexes were observed after HA exposure (p > 0.05).
Reproducibility
Intra- and inter-observer ICC was 0.84 (p < 0001) and 0.78 (p = 0001) for LASr, and 0.89 (p < 0001) and 0.80 (p < 0001) for RASr, respectively. Intra- and inter-observer ICC for other atrial strain and SR were presented in Supplementary Table 3. All measurements showed excellent or good reproducibility.
Discussion
To our knowledge, this is the first study to comprehensively evaluate the effect of short-term HA exposure on bi-atrial performance using STE. The major findings of our study were that HA exposure led to decreases in bi-atrial function, mostly in RA. After short-term HA exposure, decreased reservoir and conduit functions were observed in bi-atria, whereas decreased contractile function was observed in RA (estimated via RAEFact, RASct and pRASRct) rather than LA (Fig. 4).
Summarizing illustration. Short-term HA exposure impairs bi-atrial performance, mostly observed in RA. Especially, atrial contractile function decreases in RA rather than LA. EFtot total emptying fraction, EFpass passive emptying fraction, EFact active emptying fraction. Other abbreviations as in Fig. 1
In the present study, we observed increased LV systolic function, slightly decreased RV systolic function and altered diastolic filling pattern in both ventricles under HA exposure as previously described [12, 26, 27]. Among those physiological responses, it has been well recognized that LV systolic function adapts well to HA conditions, nevertheless RV failed as a consequence of HA-induced higher pulmonary arterial pressure [27]. Additionally, our results suggested that LV and RV intrinsic relaxation seemed unaffected by hypoxia since ventricular early filling (estimated via E′) remained unchanged after short-term HA exposure.
Regarding ventricular passive filling, atrial contraction has been commonly regarded as enhanced to overcome HA exposure-induced ventricular diastolic dysfunction in previous studies using Doppler echocardiography, which actually can’t reflect the real atrial properties [11, 28]. Recently, Sareban et al. [6, 7] presented a different perspective that LA contraction did not change, however, RA contraction increased in a few hours after a rapid ascending to HA assessed by STE. However, in the present study, we obtained a novel finding that RA contraction decreased after short-term HA exposure, which seemed not to compensate for decreased ventricular filling. Previous studies have validated that volume and strain derived parameters were preload-dependent in different degrees, of which SR appeared to be less preload-dependent [29, 30]. Indeed, Robach et al. [31] documented that plasma volume decreased within 1–3 days and fell by 13.6% after 7 days at 4350 m. Similarly, the decreased mitral and tricuspid E/E' from our data implied the loss of plasma volume after short-term HA exposure, due to its sensitivity to the changes of preload [32]. Therefore, in the present study, SR could better reflect the real atrial response to HA exposure. Besides measurement methods, the discrepancies between the present study and previous findings might be explained by differences in race, exposure duration and physical activity.
As well recognized, cardiac adaptation to HA is a comprehensive consequence of hypoxia, pulmonary vasoconstriction, sympathetic activation and hypovolemia [1, 33, 34]. Additionally, it should be acknowledged that atrium interacts with ventricle throughout the cardiac cycle. Accordingly, atrial performance under HA exposure might be multiply affected by decreased energy supply and preload, increased afterload, and altered ventricular mechanics. In this study, the subgroup analyses have clarified our hypothesis that decreased RA contractile function was linked with hypoxia and pulmonary hypertension, but not pulmonary function. As generally known, hypoxia is the initial determinant of cardiopulmonary response to high altitude and previous studies have validated hypoxia alone reduced atrial contractility [35, 36]. Additionally, the presence of TR after HA exposure was majorly secondary to hypoxic pulmonary hypertension, which caused RV excessive afterload, and ultimately conducted to RA, seeming to be the common mechanism underlying decreased RV contractility at HA. RA is a thin-wall chamber and works at lower pressure than LA under physiological condition. We speculate that it is hard for RA to adapt HA-induced pressure overload, which should be responsible for the vulnerability of RA under HA conditions. However, although HA exposure induced increased ventilation, it seemed to have little effect on atrial function. Moreover, the correlation analyses might provide additional implications as age-and BMI-related changes in RA function after ascending to HA, which need enroll larger population to verify.
Ventricular adaptation at HA has been well described, but the studies on atrial response to HA is scarce. Our findings demonstrated that short-term HA exposure impaired RA contractile function, which could provide novel evidence for HA-induced RV dysfunction. The impairment of RA function in the early stage after HA exposure might develop into HA heart disease, however whether it persists or is reversible needs longer follow-up to be revealed. Additionally, it has been widely assessed that RA function was sensitive and valuable to predict exercise capacity and clinical outcomes in non-HA-induced pulmonary arterial hypertension [37,38,39]. Indeed, physiological adaptation to high altitude has long been recognized as hypoxic pulmonary hypertension and reduced exercise capacity, especially in trekker and mountaineer. Accordingly, the impaired RA function based on this study might be linked with limited exercise capacity at HA, and individuals with worse RA function might need to reduce physical activity and even exposure duration to avoid HA related diseases. Our findings might indicate a new approach to assess and improve HA acclimatization but remains to be determined.
Limitations
Several limitations of this study should be acknowledged. The observational study was carried out in healthy adult males, thus, our results probably are not applicable to other populations. Therefore, additional populations should be included in further studies to confirm the present results. Although 2D-STE derived quantitative assessment of atrial function is feasible and sensitive, invasive cardiac catheterization and 3D echocardiography are warranted for comparison with our results. Finally, larger-scale studies and longer follow-up are needed to explore cardiac adaption to hypoxia exposure and its clinical relevance.
Conclusion
For the first time, we demonstrated that bi-atrial performance decreased following short-term HA exposure, mostly observed in RA. Especially, short-term HA exposure of healthy individuals decreased RA contractile function rather than LA, not compensating for decreased ventricular filling. Our findings may provide an important evidence to understand cardiac response to HA exposure.
References
Naeije R (2010) Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis 52(6):456–466. https://doi.org/10.1016/j.pcad.2010.03.004
Boussuges A, Molenat F, Burnet H et al (2000) Operation Everest III (Comex ’97): modifications of cardiac function secondary to altitude-induced hypoxia. An echocardiographic and Doppler study. Am J Respir Critic Care Med 161(1):264–270
Mitter SS, Shah SJ, Thomas JD (2017) A test in context: E/A and E/e’ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 69(11):1451–1464. https://doi.org/10.1016/j.jacc.2016.12.037
Thomas L, Marwick TH, Popescu BA et al (2019) Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol 73(15):1961–1977. https://doi.org/10.1016/j.jacc.2019.01.059
Rai AB, Lima E, Munir F et al (2015) Speckle tracking echocardiography of the right atrium: the neglected chamber. Clin Cardiol 38(11):692–697. https://doi.org/10.1002/clc.22438
Sareban M, Perz T, Macholz F et al (2020) Preserved right ventricular function but increased right atrial contractile demand in altitude-induced pulmonary hypertension. Int J Cardiovasc Imaging 36(6):1069–1076. https://doi.org/10.1007/s10554-020-01803-x
Sareban M, Perz T, Macholz F et al (2019) Impairment of left atrial mechanics does not contribute to the reduction in stroke volume after active ascent to 4559 m. Scand J Med Sci Sports 29(2):223–231. https://doi.org/10.1111/sms.13325
Stembridge M, Ainslie PN, Shave R (2016) Mechanisms underlying reductions in stroke volume at rest and during exercise at high altitude. Eur J Sport Sci 16(5):577–584. https://doi.org/10.1080/17461391.2015.1071876
Järvinen VM, Kupari MM, Poutanen VP et al (1996) Right and left atrial phasic volumetric function in mildly symptomatic dilated and hypertrophic cardiomyopathy: cine MR imaging assessment. Radiology 198(2):487–495
Hoit BD (2014) Left atrial size and function: role in prognosis. J Am Coll Cardiol 63(6):493–505. https://doi.org/10.1016/j.jacc.2013.10.055
Allemann Y, Rotter M, Hutter D et al (2004) Impact of acute hypoxic pulmonary hypertension on LV diastolic function in healthy mountaineers at high altitude. Am J Physiol Heart Circ Physiol 286(3):H856–H862
Maufrais C, Rupp T, Bouzat P et al (2017) Heart mechanics at high altitude: 6 days on the top of Europe. Eur Heart J Cardiovasc Imaging 18(12):1369–1377. https://doi.org/10.1093/ehjci/jew286
Todaro MC, Choudhuri I, Belohlavek M et al (2012) New echocardiographic techniques for evaluation of left atrial mechanics. Eur Heart J Cardiovasc Imaging 13(12):973–984. https://doi.org/10.1093/ehjci/jes174
Vianna-Pinton R, Moreno CA, Baxter CM et al (2009) Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 22(3):299–305. https://doi.org/10.1016/j.echo.2008.12.017
Du Bois D, Du Bois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Nutrition (Burbank, Los Angeles County, Calif) 5(5):303–311
Mitchell C, Rahko PS, Blauwet LA et al (2018) Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 32(1):1–64. https://doi.org/10.1016/j.echo.2018.06.004
Yang T, Li X, Qin J et al (2015) High altitude-induced borderline pulmonary hypertension impaired cardiorespiratory fitness in healthy young men. Int J Cardiol 181:382–388. https://doi.org/10.1016/j.ijcard.2014.12.044
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270. https://doi.org/10.1093/ehjci/jev014
Park CS, Kim YK, Song HC et al (2012) Effect of preload on left atrial function: evaluated by tissue Doppler and strain imaging. Eur Heart J 13(11):938–947. https://doi.org/10.1093/ehjci/jes069
Rudski LG, Lai WW, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713. https://doi.org/10.1016/j.echo.2010.05.010
Badesch DB, Champion HC, Sanchez MA et al (2009) Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 54(1 Suppl):S55-66. https://doi.org/10.1016/j.jacc.2009.04.011
Badano LP, Kolias TJ, Muraru D et al (2018) Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 19(6):591–600. https://doi.org/10.1093/ehjci/jey042
Kurt M, Wang J, Torre-Amione G et al (2009) Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2(1):10–15. https://doi.org/10.1161/CIRCIMAGING.108.813071
Miller MR, Hankinson J, Brusasco V et al (2005) Standardisation of spirometry. Eur Respir J 26(2):319–338. https://doi.org/10.1183/09031936.05.00034805
Hahn RT, Chandrashekhar Y (2019) Tricuspid regurgitation: a voyage of discovery. JACC Cardiovasc Imaging 12(3):572–575. https://doi.org/10.1016/j.jcmg.2019.01.010
Huez S, Retailleau K, Unger P et al (2005) Right and left ventricular adaptation to hypoxia: a tissue Doppler imaging study. Am J Physiol Heart Circ Physiol 289(4):H1391–H1398. https://doi.org/10.1152/ajpheart.00332.2005
Stembridge M, Ainslie PN, Hughes MG et al (2014) Ventricular structure, function, and mechanics at high altitude: chronic remodeling in Sherpa vs. short-term lowlander adaptation. J Appl Physiol (1985) 117(3):334–343. https://doi.org/10.1152/japplphysiol.00233.2014
Dedobbeleer C, Hadefi A, Pichon A et al (2015) Left ventricular adaptation to high altitude: speckle tracking echocardiography in lowlanders, healthy highlanders and highlanders with chronic mountain sickness. Int J Cardiovasc Imaging 31(4):743–752. https://doi.org/10.1007/s10554-015-0614-1
Genovese D, Singh A, Volpato V et al (2018) Load dependency of left atrial strain in normal subjects. J Am Soc Echocardiogr 31(11):1221–1228. https://doi.org/10.1016/j.echo.2018.07.016
Negishi K, Borowski AG, Popović ZB et al (2017) Effect of gravitational gradients on cardiac filling and performance. J Am Soc Echocardiogr 30(12):1180–1188. https://doi.org/10.1016/j.echo.2017.08.005
Robach P, Lafforgue E, Olsen NV et al (2002) Recovery of plasma volume after 1 week of exposure at 4,350 m. Pflugers Arch 444(6):821–828. https://doi.org/10.1007/s00424-002-0894-x
Nagueh SF, Middleton KJ, Kopelen HA et al (1997) Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30(6):1527–1533
Bartsch P, Gibbs JS (2007) Effect of altitude on the heart and the lungs. Circulation 116(19):2191–2202. https://doi.org/10.1161/CIRCULATIONAHA.106.650796
Holloway C, Cochlin L, Codreanu I et al (2011) Normobaric hypoxia impairs human cardiac energetics. FASEB J 25(9):3130–3135. https://doi.org/10.1096/fj.11-183426
Froldi G, Guerra L, Pandolfo L et al (1994) Phentolamine and hypoxia: modulation of contractility and alpha 1-adrenoceptors in isolated rat atria. Naunyn Schmiedebergs Arch Pharmacol 350(5):563–568. https://doi.org/10.1007/BF00173027
Monti F, Iwashiro K, Picard S et al (2000) Adenosine triphosphate-dependent potassium channel modulation and cardioplegia-induced protection of human atrial muscle in an in vitro model of myocardial stunning. J Thorac Cardiovasc Surg 119(4 Pt 1):842–848. https://doi.org/10.1016/S0022-5223(00)70022-5
Fukuda Y, Tanaka H, Ryo-Koriyama K et al (2016) Comprehensive functional assessment of right-sided heart using speckle tracking strain for patients with pulmonary hypertension. Echocardiography 33(7):1001–1008. https://doi.org/10.1111/echo.13205
Alenezi F, Mandawat A, Il’Giovine ZJ et al (2018) Clinical utility and prognostic value of right atrial function in pulmonary hypertension. Circ Cardiovasc Imaging 11(11):e006984. https://doi.org/10.1161/CIRCIMAGING.117.006984
Liu W, Wang Y, Zhou J et al (2018) The association of functional capacity with right atrial deformation in patients with pulmonary arterial hypertension: a study with two-dimensional speckle tracking. Heart Lung Circ 27(3):350–358. https://doi.org/10.1016/j.hlc.2017.02.029
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No: 81730054), Military Logistics Research Project, PLA (Grant No: BLJ18J007), the Special Health Research Project, Ministry of Health of PR China (No. 201002012) and PLA Youth Training Project for Medical Science (NO.15QNP062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, C., Liu, C., Yu, S. et al. Atrial performance in healthy subjects following high altitude exposure at 4100 m: 2D speckle-tracking strain analysis. Int J Cardiovasc Imaging 37, 1891–1902 (2021). https://doi.org/10.1007/s10554-021-02173-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02173-8