Abstract
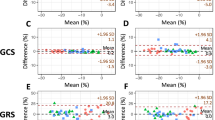
Evaluation of myocardial regional function is generally performed by visual “eyeballing” which is highly subjective. A robust quantifiable parameter of regional function is required to provide an objective, repeatable and comparable measure of myocardial performance. We aimed to evaluate the clinical utility of novel regional myocardial strain software from cardiac computed tomography (CT) datasets. 93 consecutive patients who had undergone retrospectively gated cardiac CT were evaluated by the software, which utilizes a finite element based tracking algorithm through the cardiac cycle. Circumferential (CS), longitudinal (LS) and radial (RS) strains were calculated for each of 16 myocardial segments and compared to a visual assessment, carried out by an experienced cardiologist on cine movies of standard “echo” views derived from the CT data. A subset of 37 cases was compared to speckle strain by echocardiography. The automated software performed successfully in 93/106 cases, with minimal human interaction. Peak CS, LS and RS all differentiated well between normal, hypokinetic and akinetic segments. Peak strains for akinetic segments were generally post-systolic, peaking at 50 ± 17% of the RR interval compared to 43 ± 9% for normokinetic segments. Using ROC analysis to test the ability to differentiate between normal and abnormal segments, the area under the curve was 0.84 ± 0.01 for CS, 0.80 ± 0.02 for RS and 0.68 ± 0.02 for LS. There was a moderate agreement with speckle strain. Automated 4D regional strain analysis of CT datasets shows a good correspondence to visual analysis and successfully differentiates between normal and abnormal segments, thus providing an objective quantifiable map of myocardial regional function.







Similar content being viewed by others
Abbreviations
- CS:
-
Circumferential strain
- LS:
-
Longitudinal strain
- RS:
-
Radial strain
References
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27):2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Liel-Cohen N, Tsadok Y, Beeri R, Lysyansky P, Agmon Y, Feinberg MS, Fehske W, Gilon D, Hay I, Kuperstein R, Leitman M, Deutsch L, Rosenmann D, Sagie A, Shimoni S, Vaturi M, Friedman Z, Blondheim DS (2010) A new tool for automatic assessment of segmental wall motion based on longitudinal 2D strain: a multicenter study by the Israeli Echocardiography Research Group. Circ Cardiovasc Imaging 3(1):47–53. https://doi.org/10.1161/CIRCIMAGING.108.841874
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39. https://doi.org/10.1016/j.echo.2014.10.003->
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28 (1):1-39. https://doi.org/10.1016/j.echo.2014.10.003
Collier P, Phelan D, Klein A (2017) A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol 69(8):1043–1056. https://doi.org/10.1016/j.jacc.2016.12.012
Chitiboi T, Axel L (2017) Magnetic resonance imaging of myocardial strain: a review of current approaches. J Magn Reson Imaging 46(5):1263–1280. https://doi.org/10.1002/jmri.25718
Mark DB, Berman DS, Budoff MJ, Carr JJ, Gerber TC, Hecht HS, Hlatky MA, Hodgson JM, Lauer MS, Miller JM, Morin RL, Mukherjee D, Poon M, Rubin GD, Schwartz RS (2010) ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 55(23):2663–2699. https://doi.org/10.1016/j.jacc.2009.11.013
Seneviratne SK, Truong QA, Bamberg F, Rogers IS, Shapiro MD, Schlett CL, Chae CU, Cury R, Abbara S, Brady TJ, Nagurney JT, Hoffmann U (2010) Incremental diagnostic value of regional left ventricular function over coronary assessment by cardiac computed tomography for the detection of acute coronary syndrome in patients with acute chest pain: from the ROMICAT trial. Circ Cardiovasc Imaging 3(4):375–383. https://doi.org/10.1161/CIRCIMAGING.109.892638
Lamash Y, Fischer A, Carasso S, Lessick J (2015) Strain analysis from 4-D cardiac CT image data. IEEE Trans Biomed Eng 62(2):511–521. https://doi.org/10.1109/TBME.2014.2359244
Amzulescu MS, Langet H, Saloux E, Manrique A, Boileau L, Slimani A, Allain P, Roy C, de Meester C, Pasquet A, De Craene M, Vancraeynest D, Pouleur AC, Vanoverschelde JJ, Gerber BL (2017) Head-to-head comparison of global and regional two-dimensional speckle tracking strain versus cardiac magnetic resonance tagging in a multicenter validation study. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.117.006530
Mor-Avi V, Patel MB, Maffessanti F, Singh A, Medvedofsky D, Zaidi SJ, Mediratta A, Narang A, Nazir N, Kachenoura N, Lang RM, Patel AR (2018) Fusion of three-dimensional echocardiographic regional myocardial strain with cardiac computed tomography for noninvasive evaluation of the hemodynamic impact of coronary stenosis in patients with chest pain. J Am Soc Echocardiogr 31(6):664–673. https://doi.org/10.1016/j.echo.2018.01.019
Fukui M, Xu J, Abdelkarim I, Sharbaugh MS, Thoma FW, Althouse AD, Pedrizzetti G, Cavalcante JL (2018) Global longitudinal strain assessment by computed tomography in severe aortic stenosis patients—feasibility using feature tracking analysis. J Cardiovasc Comput Tomogr. https://doi.org/10.1016/j.jcct.2018.10.020
Marwan M, Ammon F, Bittner D, Rother J, Mekkhala N, Hell M, Schuhbaeck A, Gitsioudis G, Feyrer R, Schlundt C, Achenbach S, Arnold M (2018) CT-derived left ventricular global strain in aortic valve stenosis patients: a comparative analysis pre and post transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr 12(3):240–244. https://doi.org/10.1016/j.jcct.2018.01.010
Pourmorteza A, Chen MY, van der Pals J, Arai AE, McVeigh ER (2016) Correlation of CT-based regional cardiac function (SQUEEZ) with myocardial strain calculated from tagged MRI: an experimental study. Int J Cardiovasc Imaging 32(5):817–823. https://doi.org/10.1007/s10554-015-0831-7
Tanabe Y, Kido T, Kurata A, Sawada S, Suekuni H, Yokoi T, Uetani T, Inoue K, Miyagawa M, Mochizuki T (2017) Three-dimensional maximum principal strain using cardiac computed tomography for identification of myocardial infarction. Eur Radiol 27(4):1667–1675. https://doi.org/10.1007/s00330-016-4550-9
Pourmorteza A, Schuleri KH, Herzka DA, Lardo AC, McVeigh ER (2012) A new method for cardiac computed tomography regional function assessment: stretch quantifier for endocardial engraved zones (SQUEEZ). Circ Cardiovasc Imaging 5(2):243–250. https://doi.org/10.1161/CIRCIMAGING.111.970061
Gupta V, Lantz J, Henriksson L, Engvall J, Karlsson M, Persson A, Ebbers T (2018) Automated three-dimensional tracking of the left ventricular myocardium in time-resolved and dose-modulated cardiac CT images using deformable image registration. J Cardiovasc Comput Tomogr 12(2):139–148. https://doi.org/10.1016/j.jcct.2018.01.005
Buss SJ, Schulz F, Mereles D, Hosch W, Galuschky C, Schummers G, Stapf D, Hofmann N, Giannitsis E, Hardt SE, Kauczor HU, Katus HA, Korosoglou G (2013) Quantitative analysis of left ventricular strain using cardiac computed tomography. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2013.11.026
Tavakoli V, Sahba N (2014) Cardiac motion and strain detection using 4D CT images: comparison with tagged MRI, and echocardiography. Int J Cardiovasc Imaging 30(1):175–184. https://doi.org/10.1007/s10554-013-0305-8
Tee MW, Won S, Raman FS, Yi C, Vigneault DM, Davies-Venn C, Liu S, Lardo AC, Lima JA, Noble JA, Emter CA, Bluemke DA (2015) Regional strain analysis with multidetector CT in a swine cardiomyopathy model: relationship to cardiac MR tagging and myocardial fibrosis. Radiology 277(1):88–94. https://doi.org/10.1148/radiol.2015142339
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The study was approved by the local Helsinki committee.
Informed consent
The need for patient consent was waived due to a retrospective study design.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: causes for exclusion from study
In total, we evaluated 117 patients.
-
Of these 11 were ruled out in advance for the following reasons:
-
Two part of LV cut off.
-
Four AF with significant artifacts.
-
Two severe artifacts in image due to breathing or premature beats.
-
One large LV pseudoaneurysm.
-
One low contrast.
-
One very noisy.
-
-
Thirteen were attempted but failed for the following reasons:
-
Six the software failed to initialize (unknown cause).
-
Five rejected due to poor tracking of endocardium or epicardium despite reasonable image quality (unknown reason).
-
One failed due to high noise (sd in image 90HU).
-
One failed due to low LV contrast 150HU.
-
Appendix 2: The Strain Algorithm
A finite-element-based tracking algorithm [9], optimized for 4D cardiac CT data is utilized to evaluate the regional mechanical function of the LV. The tracking algorithm is based on a deformable LV model that contains both the myocardium and the blood pool regions and that accounts for the elasticity and incompressibility physiological features of the myocardium. The algorithm uses clues for the deformation and rotation of the myocardium from the endocardial edges, trabeculae and papillary muscles. The algorithm uses a deformable LV mesh (video 1) with defined myocardium and blood pool regions and labeled endocardial and epicardial surfaces to derive the deforming forces.
Appendix 3: Running the CT Strain Software
-
1.
The CT data is loaded to the software.
-
2.
The short axis slices at end-diastole are displayed on the screen.
-
3.
The center of the LV blood pool is marked by the user in the most apical and basal slices.
-
4.
The LV blood pool and myocardium are automatically segmented for all cardiac phases, creating a 3D mesh of the LV, and an initial set of endocardial and epicardial contours are marked on the short axis slices.
-
5.
The user can edit the contours if required.
-
6.
The software calculates regional deformations between contiguous temporal phases at the subendocardium and subepicardium.
-
7.
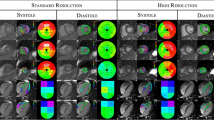
Regional deformation vectors are displayed on the short axis contours (Fig. 1, video 2 and 3) during cine mode to enable user evaluation of the success of the algorithm, especially regarding tracking of the endocardial and epicardial contours and the degree and direction of deformation relative to visual impression.
-
8.
If tracking quality is insufficient, the user has the possibility of rerunning the software after re-editing the initial endocardial and epicardial contours.
-
9.
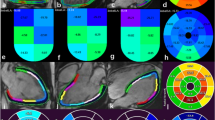
Regional strains are calculated in the circumferential, longitudinal and radial directions. Each ventricle was divided into 16 segments according to the ASE model. Segment 17 was not evaluated. Global strain was calculated as the mean of all segmental strains.
Rights and permissions
About this article
Cite this article
Peled, Z., Lamash, Y., Carasso, S. et al. Automated 4-dimensional regional myocardial strain evaluation using cardiac computed tomography. Int J Cardiovasc Imaging 36, 149–159 (2020). https://doi.org/10.1007/s10554-019-01696-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01696-5