Abstract
Speckle tracking analysis (STE) of the left ventricle offers a new method to assess left ventricular (LV) diastolic function. LV diastolic strain measurements offer a non-invasive, global and direct measure of LV diastolic function. However, there is little data on normal values and the influence of anthropomorphic factors which is crucial in clinical practice for new techniques. The aims of this study were to formulate reference values for LV diastolic strain rate, elucidate effects of age and sex on LV diastolic strain analysis and compare STE measurements with conventional LV diastolic measurements. One-hundred-forty-seven healthy subjects aged 20–72 years (≥ 28 subjects per age decade) were prospectively included (Mean age 44 ± 13.7 years, 50% female) and examined with electrocardiography and 2D-echocardiography, including speckle tracking. Left ventricular peak early diastolic strain rate (Sre) was measured in the apical windows, using STE. Men had significantly lower LV Sre values than women (1.02 ± 0.22 vs. 1.18 ± 0.23, p value < 0.001). Left ventricular Sre was inversely associated with age, with values decreasing with ageing. An inverse relation was also found with blood pressure and body surface area. Linear regression analysis showed that LV Sre was independently associated with both age and sex. A multivariable linear regression analysis for LV Sre with conventional LV diastolic variables accounted for 70.9% of the variation of LV Sre, showing good model performance. Reference values for LV Sre are reported and found to be both age- and sex-dependent. Therefore we recommend age- and sex-specific references values to be used in daily clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Left ventricular (LV) diastolic disease is increasingly being recognized as an important cause of LV heart failure. Approximately 40% of the patients present themselves with symptoms of heart failure while having a preserved ejection fraction [1]. Increased LV diastolic stiffness and reduced relaxation are thought to be the main pathophysiologic mechanism. Current guidelines on LV diastolic function recommend the use of several echocardiographic Doppler variables, including Tissue Doppler Imaging (TDI), to assess LV relaxation and predict LV filling pressures [2]. Filling pressures are dependent on loading conditions and myocardial relaxation, both are (indirectly) assessed by measuring the speed with which the myocardium lengthens during early diastole and trans mitral flow velocities. While these conventional variables are a good way to assess LV diastolic function, they do have some limitations. TDI variables are angle-dependent and measure LV relaxation only regionally, early and late mitral flow velocities are dependent on both ventricular and atrial pressure and the enlargement of the left atrium may be present in the absence of LV diastolic dysfunction. Deformation measurements derived from speckle-tracking echocardiography (STE) have been validated in human studies [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17] and offer advantages over TDI such as angle-independency and presenting global relaxation [2], offering an alternative way to assess LV diastolic dysfunction.
Reference values are of vital importance for clinical practice and possibly even more so for new techniques. A recent study published normal values for LV diastolic peak strain rate, but the influences of age and sex were not elucidated [17]. Therefore the aims of this prospective study were to assess and formulate reference values for LV diastolic strain rate in a healthy cohort of volunteers, to assess the possible effects of baseline characteristics, particularly of age, on diastolic strain rate parameters of the left ventricle, and to investigate correlations between conventional diastolic measurements and LV diastolic function assessed with strain rate analysis.
Methods
Study population
For this cross-sectional prospective cohort study, healthy volunteers were enrolled into five age-groups: 20–29, 30–39, 40–49, 50–59 and 60–72 years. Each age group had at least 28 subjects, sex was equally distributed in each group. Subjects were recruited via an advertisement for healthy subjects. Volunteers were examined at the Erasmus MC, Rotterdam and excluded if there was any (prior) cardiovascular disease, systemic disease, cardiac medication or the finding of cardiac abnormalities during examination, including signs of LV hypertrophy such as increased wall thickness. Presence of cardiovascular risk factors was also a reason for exclusion, as was renal dysfunction based on creatinine levels. Furthermore, professional athletes, morbidly obese subjects (> 40 kg/m2) and women who were pregnant or had breast implants were excluded. Details have been published earlier [18]. The study was carried out in accordance with the principles of the Declaration of Helsinki and approved by the local medical ethics committee. Informed consent was obtained from all participants.
Clinical assessment
Subjects completed a questionnaire about medical history and health status and underwent physical examination, venous blood sampling, 12-lead electrocardiography and echocardiography. Physical examination included height, weight, blood pressure, saturation and heart, lungs and abdominal findings.
Echocardiographic image acquisition
All echocardiographic studies were performed by an experienced sonographer (JM). Two-dimensional greyscale harmonic images were obtained in the left lateral decubitus position using an iE33 or EPIQ7 ultrasound system (Philips Medical Systems, Best, The Netherlands) equipped with a transthoracic broadband X5-1 matrix transducer. Standard apical 4-chamber (A4C), 2-chamber (A2C) and 3-chamber (A3C) views were obtained for strain analysis, with framerates ≥ 50 frames/s, for strain analysis.
Conventional echocardiographic measurements
The recent guideline for chamber quantification from the American Society of Echocardiography and the European Association of Cardiovascular Imaging were followed for echocardiographic measurements [19]. Similarly, the guidelines from the American Society of Echocardiography and European Association of Cardiovascular Imaging were used to assess LV diastolic function [2]. Left ventricular diastolic function was considered abnormal according to the following variables: septal e′ < 7 cm/s or lateral e′ < 10 cm/s using Tissue Doppler Imaging (TDI), an E/e′-ratio > 14, Left atrial maximum volume > 34 ml/m2 or a tricuspid regurgitation (TR) jet velocity > 2.8 m/s. All measurements were done whilst being blinded from the clinical characteristics.
Speckle tracking analysis
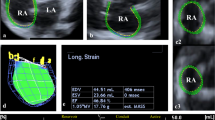
All measurements were performed by a single investigator (RG). The data sets were blinded regarding subject ID. Offline analysis was performed using QLAB 10 (Philips Medical Systems, Best, the Netherlands). Cardiac cycles were defined by the positioning of R-waves, aortic valve closure time was used to define end-systole [20] and obtained by selecting the frame of aortic valve closure on the A3C view. LV strain analysis was performed in the apical four-, three- and two-chamber views (A4C, A3C, A2C). The width of the segments was set to line up with the epicardial and endocardial border, while avoiding the epicardium. The program automatically divided the walls in segments based on the 17 segment model and tracked these on a frame-by-frame basis [21]. When tracking was suboptimal, the borders were adjusted. Segments with persistently inadequate tracking were excluded for analysis. Also if three or more segments were inadequate the measurements were excluded. Peak strain rate was defined as the peak value during early diastole. For each segment the LV early diastolic strain rate peak (LV Sre) was assessed. The average of LV Sre was calculated for each view and for the entire left ventricle by averaging the segmental LV Sre values (Fig. 1).
Statistical analysis
Normal distribution was checked visually using histograms and with the Shapiro–Wilk test. Depending on data distribution, continuous data are presented as mean ± standard deviation (SD) or median [Q1–Q3]. Categorical data are presented as frequencies and percentages. For comparison the paired t-test, Student’s t-test, Mann-Whitney-U test, χ2-test, Fisher exact test was used as was appropriate. Linear regression analysis was used to assess correlations between baseline characteristics and strain variables. A second model analysis was done to assess correlations between conventional diastolic variables and LV early peak diastolic strain rate. Variables that reached p < 0.001 and did not show collinearity with other variables were included in a multivariable model. In case of collinearity (Pearson r > 0.6), the variable with the highest correlation was included. Statistical analysis was done using the Statistical Package for Social Science version 21 (IBM DPDD Statistics for Windows, Armonk, New York, USA). Statistical test were considered significant with a p value of ≤ 0.05.
Results
Study population
Of the 155 eligible volunteers, 147 were included in the study. Reasons for exclusion were breast implants (n = 2), valvular pathology (n = 2), surgically closed duct (n = 1), hypertension (n = 1), morbid obesity (n = 1) and right bundle branch block on ECG (n = 1). Baseline characteristics of the study population per age group are shown in Table 1. Overall, the mean age was 44.6 SD 13.8 years and 50% was female (also equally distributed per age-group). Median systolic and diastolic blood pressure was 126 (116–133) and 80 (75–85) mmHg respectively. Of the 147 volunteers included, 135 (92%) had normal diastolic function and 9 (6%) had an indeterminate diastolic function, according to the current guideline [2].
Diastolic strain rate
Strain rate analysis was highly feasible, it was possible to measure global strain rate in 98.0% of the patients in the A4C-view, 97.3% of the A2C-views and 95.9% of the A3C-views. Of the entire cohort the mean global LV Sre values of each view were: A4C Sre 1.14 SD 0.29 s−1, A2C Sre: 1.09 SD 0.25 s−1 and A3C Sre 1.09 SD 0.25 s−1. The mean LV global Sre was 1.11 SD 0.24 s−1. The LV Sre values per age group are presented in Table 2 and supplemental Table 1. Linear regression analysis with age as a continuous variable showed that every segment was significantly inversely related to age (Fig. 2). Of the three views, LV Sre measured in the A4C view correlated strongest with age (r = − 0.561, p value < 0.001). Besides having the strongest decrease with age, the strain rate values in the different segments of the A4C-view were also the highest.
Influences of baseline characteristics
Left ventricular Sre values were sex-dependent, with lower values for males in every segment (Table 3 and Supplemental Table 2). We further explored sex-dependency by also stratifying for age (Fig. 3). This figure shows that values for females were higher across all age groups, and there seems to be a sharper decline in LV diastolic function after the 5th decade.
Univariable linear regression analysis was performed between baseline characteristics and LV Sre (Table 4). Both a higher systolic and diastolic blood pressure led to a linear decrease in LV Sre values. Lower LV volumes (indexed for BSA) were associated with a higher LV Sre.
Table 4 also shows a stepwise multivariable linear regression analysis of baseline characteristics and LV global Sre. This shows that LV global Sre is independently correlated with age, sex and systolic blood pressure.
A second multivariable linear regression analysis was performed (Table 4) which included several conventional LV diastolic variables: E-wave velocity, deceleration time, e′, and also LV end diastolic volume indexed for BSA, LV ejection fraction (EF) and LV global longitudinal strain (GLS). The adjusted r2 was 0.709, meaning 70.9% of the variation of LV Sre can be predicted with this model. E-wave velocity and e′ were independently correlated with LV SRe, as were the systolic indices LV EF and LV GLS.
Discussion
This study demonstrates a clear relation between LV early diastolic peak strain rate and age. There is a clear decrease of LV Sre with ageing, which is especially notable after the fifth decade. The second important finding is that there are significant sex-dependent differences, with males having consistently lower LV Sre values than females.
Reference values are crucial in clinical practice to distinguish normal from abnormal. A previous study determined the lower limit of normal of LV Sre in a large population of healthy subjects [17] and evaluated the added value to conventional assessment of LV diastolic function. The lower limit of normal in their cohort was 1.00 s−1, based on their mean value of 1.56–2SD. The mean in our cohort was notably lower, namely 1.10 ± 0.24, which would result in a lower limit of normal of 0.62 s−1. A possible explanation for this difference may be the higher mean age of our study population (44.6 SD 13.7 in our study vs. 36.5 SD 12.8 years in the study of Morris et al [17]).
It has been widely recognized that with age, LV diastolic function decreases even in the absence of cardiovascular disease [2]. It considered to be either a part of the ageing process, or as a physiological process, where the left ventricle is becoming slightly stiffer. The added value over for instance Morris et al. [17] is that this study is the first to demonstrate this ageing process is also measurable when assessing LV diastolic function with STE. A closer inspection shows a sharper decrease in LV diastolic function at the onset of the fifth decade, as can be seen in Fig. 1. The combination of a younger study population and this sharper decline at the fifth decade may explain why Morris et al. [17] found no age-dependency. Though no invasive measurements were performed, none of the volunteers had LV diastolic dysfunction according to current guidelines.
Though the current guidelines do not mention differences in LV diastolic function between men and women, we found consistently lower LV diastolic Sre values in men. The difference in mean LV Sre between men and women was 0.16 s−1. Marwick et al. reported that sex is independently correlated with LV diastolic Sre [22], and Morris et al. found modestly though significantly lower values in men [17]. These studies all suggest that there is a need for sex-specific LV Sre values.
A few studies have looked at LV Sre in populations with a wide variety of diseases and conditions, but only a few studies have actually looked at LV Sre in a cohort of healthy subjects. Besides age and sex, body size, blood pressure and QRS-duration were correlated with LV diastolic Sre. We also expected heart rate to correlate with LV diastolic function, but this was not the case in our study, most likely due to the lack of variation in heart rate (62 SD 10 bpm in our study population).
The current algorithm to assess LV diastolic function is very good but relies on variables which have some limitations; the early diastolic peak velocity of the mitral annulus assessed with TDI which is angle-dependent, maximum LA volume can be enlarged is in the absence of LV diastolic dysfunction as it is an indirect measure of LV diastolic function, and E- and A-wave velocities are dependent on LV and left atrial filling pressures [2]. This makes them less appropriate for instance in patients with a dilated left ventricle [23], patients with regional dysfunction [24] and patients with atrial fibrillation [25]. In our study population consisting of healthy individuals feasibility of conventional LV diastolic variables was very good. Recently the added value of LV Sre on top of the current algorithm was examined and revealed that LV Sre has indeed added value to diagnose LV diastolic dysfunction [17]. We argue that the use of age- and sex-specific values can improve this algorithm even further.
The results also showed that LV systolic function was independently associated with LV Sre. A better systolic function logically leads to better diastolic function [2] (in the absence of cardiac disease) as this leads to better diastolic function as can be witnessed by an enhanced twisting and untwisting motion of the left ventricle [26].
Limitations
Between-vendor variability is often mentioned as a limitation for clinical application of strain analysis. In this study, we only used Philips ultrasound equipment and QLAB software. However, after the implementation of the standardization process, the variability of strain measurements in later developed versions of major ultrasound manufacturers have been reduced, making the reported values by us more widely applicable nowadays. Several studies showed that differences between vendors are very small, albeit statistically significant, though one could argue if these differences are clinically relevant [27, 28]. Due to the relatively small sample size, conclusions should be drawn with caution.
Conclusion
This study presents reference values for LV diastolic early diastolic strain rate peak based on a cohort of healthy individuals with a large variation in age and revealed that LV diastolic function assessed with STE, is age- and sex-dependent. We therefore strongly advise sex- and age-specific reference values for LV diastolic strain measurements with STE in daily clinical practice.
References
Gladden JD, Chaanine AH, Redfield MM (2018) Heart failure with preserved ejection fraction. Annu Rev Med 69:65–79
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 17(12):1321–1360
Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF (2007) Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation 115(11):1376–1383
Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N (2008) Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol 101(10):1504–1509
Amundsen BH, Crosby J, Steen PA, Torp H, Slordahl SA, Stoylen A (2009) Regional myocardial long-axis strain and strain rate measured by different tissue Doppler and speckle tracking echocardiography methods: a comparison with tagged magnetic resonance imaging. Eur J Echocardiogr 10(2):229–237
Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M et al (2009) Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 104(10):1398–1401
Cheung YF, Liang XC, Chan GC, Wong SJ, Ha SY (2010) Myocardial deformation in patients with Beta-thalassemia major: a speckle tracking echocardiographic study. Echocardiography 27(3):253–259
Mu Y, Qin C, Wang C, Huojiaabudula G (2010) Two-dimensional ultrasound speckle tracking imaging in evaluation of early changes in left ventricular diastolic function in patients with essential hypertension. Echocardiography 27(2):146–154
Garceau P, Carasso S, Woo A, Overgaard C, Schwartz L, Rakowski H (2012) Evaluation of left ventricular relaxation and filling pressures in obstructive hypertrophic cardiomyopathy: comparison between invasive hemodynamics and two-dimensional speckle tracking. Echocardiography 29(8):934–942
de Bie MK, Ajmone Marsan N, Gaasbeek A, Bax JJ, Groeneveld M, Gabreels BA et al (2012) Left ventricular diastolic dysfunction in dialysis patients assessed by novel speckle tracking strain rate analysis: prevalence and determinants. Int J Nephrol 2012:963504
Uraizee I, Cheng S, Hung CL, Verma A, Thomas JD, Zile MR et al (2013) Relation of N-terminal pro-B-type natriuretic peptide with diastolic function in hypertensive heart disease. Am J Hypertens 26(10):1234–1241
Chen S, Yuan J, Qiao S, Duan F, Zhang J, Wang H (2014) Evaluation of left ventricular diastolic function by global strain rate imaging in patients with obstructive hypertrophic cardiomyopathy: a simultaneous speckle tracking echocardiography and cardiac catheterization study. Echocardiography 31(5):615–622
Liu D, Hu K, Stork S, Herrmann S, Kramer B, Cikes M et al (2014) Predictive value of assessing diastolic strain rate on survival in cardiac amyloidosis patients with preserved ejection fraction. PLoS ONE 9(12):e115910
Goebel B, Haugaa KH, Meyer K, Otto S, Jung C, Lauten A et al (2014) Early diastolic strain rate predicts response to heart failure therapy in patients with dilated cardiomyopathy. Int J Cardiovasc Imaging 30(3):505–513
Cordero-Reyes AM, Youker K, Estep JD, Torre-Amione G, Nagueh SF (2014) Molecular and cellular correlates of cardiac function in end-stage DCM: a study using speckle tracking echocardiography. JACC Cardiovasc Imaging 7(5):441–452
Panoulas VF, Sulemane S, Konstantinou K, Bratsas A, Elliott SJ, Dawson D et al (2015) Early detection of subclinical left ventricular myocardial dysfunction in patients with chronic kidney disease. Eur Heart J Cardiovasc Imaging 16(5):539–548
Morris DA, Takeuchi M, Nakatani S, Otsuji Y, Belyavskiy E, Aravind Kumar R et al (2017) Lower limit of normality and clinical relevance of left ventricular early diastolic strain rate for the detection of left ventricular diastolic dysfunction. Eur Heart J Cardiovasc Imaging 19(8):905–915
Menting ME, McGhie JS, Koopman LP, Vletter WB, Helbing WA, van den Bosch AE et al (2016) Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography 33(11):1665–1675
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G et al (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12(3):167–205
Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R et al (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 16(1):1–11
Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P et al (2009) Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging 2(1):80–84
Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH (2009) Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation 119(1):62–70
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM et al (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794
Nagueh SF, Kopelen HA, Quinones MA (1996) Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation 94(9):2138–2145
Notomi Y, Martin-Miklovic MG, Oryszak SJ, Shiota T, Deserranno D, Popovic ZB et al (2006) Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation 113(21):2524–2533
Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU (2015) Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr 28(10):1171–1181
Unlu S, Mirea O, Duchenne J, Pagourelias ED, Bezy S, Thomas JD et al (2018) Comparison of feasibility, accuracy, and reproducibility of layer-specific global longitudinal strain measurements among five different vendors: a report from the EACVI-ASE strain standardization task force. J Am Soc Echocardiogr 31(3):374–380
Funding
This study has been sponsored by a grant from the Erasmus Thorax Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.W.J. van Grootel, R.M. Kauling, M.E. Menting, J. McGhie, J.W. Roos-Hesselink, and A.E. van den Bosch declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Grootel, R.W.J., Kauling, R.M., Menting, M.E. et al. Influence of age and sex on left ventricular diastolic strain analysis. Int J Cardiovasc Imaging 35, 491–498 (2019). https://doi.org/10.1007/s10554-018-1480-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1480-4