Abstract
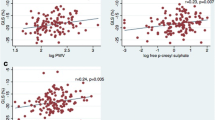
Emerging cardiovascular biomarkers, such as speckle tracking echocardiography (STE) and aortic pulse wave velocity (aPWV), have recently demonstrated the presence of subclinical left ventricular dysfunction and arterial stiffening in patients with chronic kidney disease (CKD) and no previous cardiovascular history. However, limited information exists on the prognostic impact of these biomarkers. We aimed to investigate whether STE and aPWV predict major adverse cardiac events (MACE) in this patient population. In this cohort study we prospectively analysed 106 CKD patients with no overt cardiovascular disease (CVD) and normal left ventricular ejection fraction. Cardiac deformation was measured using STE while aPWV was measured using arterial tonometry. The primary end-point was the composite of all-cause mortality, acute coronary syndrome, stable angina requiring revascularization (either using percutaneous coronary intervention or coronary artery bypass surgery), hospitalization for heart failure and stroke. Over a median follow up period of 49 months (interquartile range 11–63 months), 26 patients (24.5%) reached the primary endpoint. In a multivariable Cox hazards model, global longitudinal strain (GLS) (HR 1.12, 95% CI 1.02–1.29, p = 0.041) and aPWV (HR 1.31, 95% CI 1.05–1.41, p = 0.021) were significant, independent predictors of MACE. GLS and aPWV independently predict MACE in CKD patients with normal EF and no clinically overt CVD.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) patients are a group at significantly increased risk of cardiovascular (CV) events, as a result of prolonged exposure to an ever-growing array of traditional and non-traditional risk factors [1]. Most risk factors exert their influence on the myocardium and arterial wall itself, causing collateral damage to organs when circulatory function is altered sufficiently to result in clinical disease [2]. The process of circulatory damage occurs over many years, and although outcomes [such as stroke or myocardial infarction (MI)] occur at identifiable discrete times, it is much more difficult to cross-sectionally assess the influence of established biomarkers of CV risk, such as glucose or cholesterol, without some surrogate for cardiovascular outcomes [3]. In the last few years a number of surrogate markers of subclinical myocardial and arterial dysfunction have emerged to quantify the degree of subclinical CV impairment. These surrogate markers are particularly relevant to CKD, a population at increased risk of CV disease (CVD) [1].
Conventional echocardiographic measures of left ventricular (LV) geometry, structure and function are significant predictors of outcomes in the CKD population. Prevalence of LV hypertrophy (LVH) [4, 5], diastolic dysfunction (DD) and LV systolic impairment [6–9], predict adverse cardiovascular outcomes and increased and mortality. Although of importance the bulk of echocardiographic studies have been performed in the presence of major structural and functional cardiac abnormalities. Recently, speckle-tracking analysis, a novel echocardiographic technique that assesses intrinsic deformation (strain) of the myocardium has been proposed as a sensitive tool to detect myocardial dysfunction before a decrease in LV ejection fraction (LVEF) occurs [10]. Additionally, speckle-tracking echocardiography (STE) has been validated against sonomicrometry and cardiac magnetic resonance imaging (cMRI) [11]. Currently, some authors have used STE to investigate the predictive value of subclinical myocardial disease in both end stage renal failure (ESRF) [12] and CKD [13]. Although these studies provide some insight into the relationship between subclinical myocardial disease and CV mortality they are limited by the fact that novel elements of deformation, such as strain rate (SR) or LV twist, were not studied. In addition large proportions of the population had previous cardiac events, confounding the significance of STE parameters in asymptomatic patients without previous cardiovascular history.
Previous studies have also shown that aortic stiffness as measured by aortic pulse wave velocity (aPWV) predicts cardiovascular mortality in patients with CKD [14] and ESRF [15]. Furthermore, recent work demonstrated that arterial remodelling is associated with CKD progression [16] and that in advanced CKD, stiffness parameters can predict CKD progression [17]. Despite this, no prospective data is available analysing the role of aPWV in predicting major adverse CV events in CKD patients with normal LVEF.
The current study hypothesized that subclinical markers of myocardial (STE) and vascular (aPWV) disease can provide important prognostic value in predicting major adverse cardiac events (MACE) in CKD patients (of all stages) with no overt CVD and normal LVEF by conventional echocardiography.
Methods
Study population
A prospective, observational cohort study was conducted involving 121 consecutive patients, recruited from outpatient nephrology clinics at Hammersmith and Charing Cross Hospitals, Imperial College Healthcare NHS, London, UK. Written informed consent was obtained from all participants and the study was approved by the UK National Research Ethics Committee Service (REC 10/H0704/81). The study conforms to the principles outlined in the Declaration of Helsinki. Patients on haemodialysis, or those with clinical or echocardiographic evidence of LV systolic dysfunction (Simpson biplane LVEF < 55%), presence of regional wall motion abnormalities, significant valvular abnormalities (moderate or severe), presence of atrial fibrillation or flutter, known pulmonary hypertension, congenital heart disease, cardiomyopathy, pericardial disease, or inadequate echocardiographic acoustic windows were excluded from this study.
All patients were asymptomatic from a cardiovascular point of view, and had normal systolic function, as assessed by conventional echocardiography. We carefully reviewed the clinical notes for all patients and any patient displaying evidence or signs of heart failure was excluded from the study.
Study protocol
CKD etiology, cardiovascular risk factors, and detailed drug history were recorded. Bio-chemical results were obtained from the most recent renal clinic review, provided that there was no evidence of superimposed acute kidney injury during the time of blood sampling. The value of estimated glomerular filtration rate (eGFR) was calculated using the four-variable equation in the Modification of Diet in Renal Disease (MDRD) study [18].
Follow-up
All participants were followed up for a median time of 49 months (interquartile range 11–63 months). The primary end-point was a composite of all-cause mortality, acute coronary syndrome, stable angina requiring revascularization [either using percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG)], hospitalization for heart failure and stroke. Deaths were identified from the office of national statistics (ONS). All patients were followed up either in outpatient clinics or by telephone conversation.
Conventional echocardiography
All echocardiographic parameters were obtained using the VIVD 7 echocardiographic machine (GE healthcare, Little Chalfont, United Kingdom). The images obtained were stored and subsequently analyzed offline in EchoPac version 12 (GE Healthcare) by a single, accredited investigator blinded to all baseline data.
LVEF was obtained using the modified Simpson’s rule [19]. LV dimensions and LV wall thickness were measured in the parasternal long axis view [19]. Left atrial (LA) volume indexed to body surface area (LAVI) was measured using the LA disk summation algorithm [19]. LV mass was estimated using the Deveraux formula [20] and posteriorly indexed to the body surface area to obtain the left ventricular mass index (LVMI). Pulse wave Doppler in the 4-chamber view was used to assess mitral inflow patterns (E/A ratio, deceleration time). Pulsed wave tissue Doppler imaging of both the lateral and septal walls was performed to measure early diastolic e tissue velocity (e′). To assess LV filling pressures we calculated E/e′ [21]. The algorithm to detect and classify the severity of LV diastolic dysfunction was based in the current recommendations for the evaluation of LV diastolic function by Echocardiography [21].
Speckle tracking echocardiography
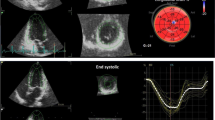
Customized software performed the speckle tracking analysis for VIVID 7 (2D-strain EchoPac PC v.7.0.1, GE Healthcare Little Chalfont, United Kingdom). Global longitudinal strain (GLS) Fig. 1 and strain rate (GLSr) were obtained from the three apical views. Global circumferential strain (GCS) and strain rate (GCSr) values were obtained from the basal, mid, and apical short-axis planes. Global strain values were calculated from the average of the 18 segments in the longitudinal or circumferential planes. LV twist was calculated as the net difference in peak systolic rotational strain between the six basal and six apical segments. The early and late diastolic SR parameters SRe and SRa were calculated from 18 segments in the longitudinal direction. A SRe/SRa ratio <1.1 was regarded as an index of altered segmental relaxation—segmental DD [22]. The total number of segmental DD was calculated for all participants.
Left ventricular global longitudinal strain (GLS) in a chronic kidney disease patient who did not reach the primary endpoint (MACE). GLS represents the average value of the peak systolic longitudinal strain of the three apical views (four-chamber, two-chamber, and long-axis), using a 17-segment model. The bottom right graph depicts a bulls-eye plot with regional (each segment) and global (average) strain. Nearly normal strain is represented in red, whereas impaired strain is represented in shades of red/pink, with areas of most impaired strain depicted in very light pink or blue. In this patient LV GLS was within normal limits at −21.2%. AVC aortic valve closure, APLAX apical long axis, 2Ch two-chamber, 4Ch four-chamber
Vascular stiffness
On the same day, approximately 10 min after performing the echocardiogram, blood pressure was measured with the subjects still in a supine position. Pressure waveforms were recorded on the carotid and femoral arteries using applanation tonometry [23]. Carotid-femoral aPWV Fig. 2 was calculated using a commercially available device (SphygmoCor, Pulse Wave Analysis System, AtCor Medical), with a high-fidelity Millar strain-gauge transducer (Millar Instruments, Houston, TX) as described previously [24]. Two separate operators collected the measurements with coefficient of variation of <10%.
Aortic PWV was measured using the foot-to-foot velocity method from various waveforms. These are usually obtained, transcutaneously at the common carotid artery and the femoral artery (i.e. ‘carotid-femoral’ PWV − ΔL), and the time delay (Δt or transit time) measured between the feet of the two waveforms
Statistical analysis
SPSS V22 (IBM Corp. Armonk, NY, 2015) was used for statistical analysis. Normality of the variables assessed was tested using the Kolmogorov–Smirnov test. Data are presented as mean ± SD. Continuous variables were compared using a Student’s t test and categorical variables using the Chi square or Fisher’s exact test as appropriate. Estimated primary outcome free survival in different groups was calculated using the Kaplan Meier method, and comparisons were made using the log-rank test. Multivariate Cox proportional hazards regression analysis was performed to identify independent predictors of the primary outcome. Two tailed p values of <0.05 were considered statistically significant.
Results
Following the exclusion of patients with poor endocardial definition (9) and pre-existing structural heart disease (6) at baseline echocardiography, the final patient population included 106 patients.
Reproducibility
We previously demonstrated excellent intra and inter-observer agreement of STE and aPWV values between individuals [25].
Baseline clinical and imaging characteristics according to the primary endpoint
Median follow up was 49 months (interquartile range 11–63 months). Overall 26 patients (24.5%) reached the primary end point. During the follow up period, five deaths (4.7%) (3 due to heart failure, one sudden death and one due to sepsis), four ST elevation MI (3.8%), two non-ST elevation MI (1.9%), three admissions for urgent PCI (2.8%), five admissions for an elective PCI (4.7%), three CABG revascularizations (2.8%), one unstable angina [leading to CABG revascularization (0.9%)], one admission for heart failure (0.9%) and two strokes (1.9%) occurred.
The baseline clinical and imaging characteristics are shown in Table 1. There were no significant differences in demographic characteristics between patients suffering MACE and those who did not. Patients who suffered MACE had higher BMI (27.5 vs. 24.3, p = 0.04), lower eGFR (23.2 vs. 47.5, p < 0.001) and a higher percentage of hypertensive heart disease (82% vs. 65% p = 0.01). Baseline GLS (−16.8% vs. −19.1%, p < 0.001) and GLSr (−0.78 vs. −1.03 m.s, p = 0.01) were significantly decreased in patients suffering MACE, while LV twist (19.3° vs. 16.3° p = 0.05) and aPWV (11.5 vs. 8.6 m/s p < 0.001) were significantly higher.
Univariate and multivariate predictors of outcome
As shown in Table 2, univariate Cox proportional hazards modelling demonstrated that a higher LVMI [hazard ratio (HR) 1.078, 95% confidence interval (CI) 1.002–1.035, p = 0.027], increased E/e′ (HR 1.177, 95% CI 1.091–1.270, p < 0.001), impaired GLS (HR 1.239, 95% CI 1.088–1.410, p = 0.01) and GLSr (HR 3.369, 95% CI 1.710–6.638, p < 0.001) and a higher aPWV (HR 1.331, 95% CI 1.089–1.692, p = 0.001) were significantly associated with the primary end−point.
In a multivariable analysis (Table 2), GLS (HR 1.12, 95% CI 1.02–1.29, p = 0.041) and aPWV (HR 1.31, 95% CI 1.05–1.41, p = 0.021) remained significantly and independently associated with the primary end-point.
From all the imaging parameters, we found that GLS with a cut-off value of −17.7% (72.3% sensitivity, 70.3% specificity), and aPWV with a cut-off value of 10.2 m/s (76.1% sensitivity and 69.6% specificity) had the best discriminatory power to predict the occurrence of the primary outcome (Table 3). Furthermore, patients with a GLS greater than −17.7% (log rank p = 0.027) and aPWV of 10.2 m/s or more (log rank p = 0.019) had a reduced estimated MACE free survival at 49 months follow up (Fig. 3).
Event free survival according GLS (upper graph) and aPWV (lower graph) cut−off values. Kaplan–Meier time to primary endpoint curves stratified according to a GLS and aPWV of −17.7% and 10.2 m/s respectively. Patients with GLS more then (less negative) −17.7% and aPWV more than 10.2 m/s were more likely to reach a primary endpoint at a median follow up of 49 months ± 9 months when compared with those with a GLS less then (more negative) −17.7% and aPWV of less then 10.2 m/s
Sub-analysis according to CKD stages
Cox univariate regression sub-analysis between CKD groups demonstrated that more advanced CKD (stages 4 and 5) had more outcomes when compared with earlier stages CKD (17% vs. 7.5% plog rank = 0.001). Aortic PWV was a significant predictor in later CKD stages (HR 1.48, 95% CI 1.03–1.79, p = 0.022). Although we didn’t find any statistical significance from the speckle tracking parameters between the groups we saw a considerable trend toward significance with GLS (HR 1.189, 95% CI 0.971–1.383, p = 0.071) Table 4.
Discussion
This prospective, cohort study demonstrates that GLS and aPWV are significant, independent predictors of MACE in CKD patients with no clinical and conventional echocardiographic evidence of CVD.
Previous observational and prospective studies have demonstrated that in the general population, GLS provides incremental value over conventional imaging [26] and biochemical measurements [27] in predicting survival following MI [27], cardiac surgery [28] or heart failure [29]. Few studies have addressed the relationship between GLS and CV mortality in CKD patients. In 2014, a single-centre study by Krishnasamy et al. [13] examined the association between GLS and all-cause mortality in 447 CKD patients. In the 5-year follow-up period, GLS was a significant independent predictor of mortality (HR 1.08, 95% CI 1.01–1.15) [13]. More recently, Kramann et al. demonstrated the ability of strain parameters to identify uremic cardiomyopathy and predict CV mortality in dialysis patients [12]. Of interest, however, a large portion of the patients included in the afore-mentioned studies had already suffered a cardiovascular event, hence confounding the significance of their results in patients with no clinical cardiovascular disease. Additionally, GLS has been shown to be sensitive to loading conditions, especially afterload [30] consequently, variations in GLS measurements due to loading conditions occur in dialysis patients and results from such studies should be viewed with caution. Lastly, novel deformation parameters (i.e. LV twist or strain rate) were not assessed. Speckle tracking echocardiography allows direct assessment of myocardial muscle shortening and lengthening by assessing not only strain, but also strain rate and LV rotation/twist [31].
In the current study we used a wide range of subclinical cardiovascular measures such as strain, strain rate, LV twist, number of segments with DD or aPWV, in an attempt to identify subclinical prognostic information. Our cohort encompassed CKD patients at all stages, with no previous history of CVD, while dialysis patients were excluded. Our results demonstrated a significant, independent relationship between impaired GLS and MACE in CKD patients with no CVD and normal EF on conventional echocardiography. In our cohort a GLS of −17.7% or less had a good discriminative power (sensitive and specificity) to predict MACE. Another interesting finding of the current study is that, at baseline, patients that reached our composite primary endpoint (MACE) had significantly impaired GLS compared to patients who did not suffer MACE, even though LVEF was similar in both groups. This fact suggests that GLS may be able to detect CKD-related myocardial changes early in its course, as it primarily evaluates the function of subendocardial longitudinal fibres that are more sensitive to cardiac injury [32]. In fact, animal and human studies support the idea that changes in myocardial composition and function that are fuelled by traditional (hypertension, diabetes) and non-traditional (oxidative stress, inflammation, thrombosis) risk factors occur early in the course of the disease, prior to the onset of clinical manifestations [33, 34], supporting the hypothesis that GLS could act as a CV biomarker of outcome in the high risk cohort of CKD patients. The majority of deaths in our cohort were caused by heart failure (3/5). In patients with heart failure, GLS was shown to be a superior predictor of cardiac events and all-cause mortality compared to EF [29, 35]. GLS is impaired in heart failure patients with preserved ejection fraction (HFpEF) [36, 37]. This relates to the fact that subendocardial function is likely to be impaired in diastolic heart failure patients while epicardial/transmural function remains preserved which explains the preserved LV ejection fraction (LVEF). Abnormal longitudinal function in this cohort can be detected at an earlier stage by examining subendocardial function, by cardiac magnetic ressonace [38] or by GLS measurement [37]. This suggests that GLS could act as more sensitive prognostic biomarker for HFpEF patients.
None of the remaining deformation parameters were significant predictors of CV events. Due to the derivation of strain, SR is inherently noisier than strain, and previous correlations between MRI tagging and STE were poorer for SR when compared with strain [39], which may partly explain our findings. It is also known that GCS may remain relatively preserved to compensate for cardiac function when longitudinal function begins to become impaired in patients with early stage heart failure [40]. LV hypertrophy, which is observed even in early stage CKD [33], is mainly caused by hypertrophic responses in the mid-myocardial layers that are predominantly circumferentially orientated. This compensates for the reduction in longitudinal function and may explain preserved LVEF and GCS [41] in our cohort.
LV rotational mechanics are altered with advancing age and various medical conditions such as MI, hypertension, diabetes mellitus and dilated cardiomyopathies [42–45], however, very little is known about LV twist in CKD. The LV twists along its long axis during the cardiac cycle as the result of counter clockwise and clockwise rotation of the apex and the base, respectively, in systole and vice versa in diastole [46]. This twisting motion or torsion has been attributed to interaction between the epicardial and endocardial helical myocardial fibre couples [31]. Our group previously identified, for the first time LV twist as a subclinical marker of early renal impairment [47], nevertheless in the current study no significant independent association between twist and outcomes was identified. We found, however, that patients who suffered MACE had significantly higher LV twist, supporting the growing evidence that LV rotational mechanics can potentially be used as an early sensitive marker of myocardial disease. Larger outcome studies are required to assess whether this parameter may be useful for prediction of cardiac events in CKD patients.
From the conventional echocardiographic parameters, only E/e′ (average) was a significant, independent predictor of MACE in our cohort. LV diastolic dysfunction, characterised by abnormalities of ventricular filling, including decreased diastolic distensibility and impaired relaxation, is a very common structural abnormality in patients with CKD [48]. E/e′ has been considered a robust marker in the prediction of LV filling pressure in a variety of conditions [49] and it has been validated against invasive methods for LV filling pressure assessment [50]. Our findings are in line with previous studies suggesting that E/e′ ratio, which is elevated in CKD and ESRF patients [51], predicts mortality and cardiovascular events in CKD patients with diastolic dysfunction [52, 53].
Arterial remodelling is known to be present even in the early stages of CKD. Compared with normotensive and hypertensive controls, patients with CKD stages 2–5 had significantly larger internal carotid artery diameters but comparable intima-media thickness, resulting in significantly increased circumferential wall stress [54]. When it comes to CV events, our study complements the findings of the Nephrotest study [55] regarding the important relationship between aPWV and all cause mortality and non-fatal CV events in CKD. They identified aPWV as an independent predictor of all-cause mortality and fatal or non-fatal CV events in 439 patients with CKD stages 3–5. However, 28% of their population had a history of CV disease. The present study further extends these findings in a cohort with no clinical evidence of CVD. In our study, patients with an aPWV of 10.2 m/s or more had reduced estimated MACE free survival at median follow up of 49 months (57.8% vs. 91.4%, plog rank = 0.019).
We also aimed to perform a sub analysis to assess predictors of outcome in patients with early (stages 1–3) versus advanced (stages 4,5) CKD. A few authors already established that cardiac and arterial changes start to develop early in the uremic milieu [14, 56]. In our paper, however, the absence of significant predictors of outcome in CKD stages 1–3 can be explained by the low number of outcomes in this sub-group when compared with CKD stages 4–5 (7.5% vs. 17%, plog rank = 0.001). Nevertheless, in CKD stages 4–5, we found that aPWV is the most robust parameter to predict MACE in our cohort, which is in line with previous research [55]. In statistical terms, GLS was not a significant predictor in either group. Larger outcome studies are necessary to study the value of GLS within CKD groups.
The current study presents a number of strengths. It includes a comprehensive assessment of subclinical myocardial and vascular parameters in an asymptomatic from the cardiovascular point of view CKD cohort with a long follow-up period (49 ± 9 months). However the results of this study may require careful interpretation due to some limitations. (1) Although STE measurements as strain or LV twist have been validated against sonomicrometry and CMR, vendor reproducibility [57], the need for optimal image quality and time constraint are currently limiting STE use in routine clinical practice; (2) The presence of significant coronary epicardial artery disease was not ruled in all patients. All patients’ from our cohort were clinically asymptomatic with normal LVEF and no regional wall motion abnormalities by conventional echocardiography. Therefore they did not fulfill the criteria to be subjected to any invasive (coronary angiography) or functional testing. However, within our cohort in a subset of 20 pre-dialysis patients who underwent elective invasive angiography as part of their pre-transplant assessment (following recruitment to our study), the vast majority of patients (N = 19) had no significant disease in their epicardial arteries. Additionally, 12 additional CDK stage 4–5 patients had a Dobutamine Stress Echocardiogram as part of their pre transplant assessment. Eleven patients had a negative test for ischaemia and 1 patient had blunted chronothropic response and thus did not achieve 85% of maximal predicted heart rate despite the use of atropine. From a total 32 patients that underwent functional/invasive testing to exclude the presence of significant coronary/myocardial disease, 30 (94%) had no significant epicardial disease/myocardial ischemia. This fact supports the hypothesis that microvascular ischaemia may account for the GLS patterns observed in the current CKD population. Interesting to notice that despite the absence of myocardial ischaemia and normal epicardial arteries 7 of these patients suffered MACE. This is line with current literature that suggests that non-traditional risk factors—such as inflammation, oxidative stress, and elevated lipoprotein levels—have been shown to have a role in promoting CVD risk [58, 59]. (3) Even though we adjusted for a large number of patient characteristics, the possibility of residual confounding effects cannot be excluded.
Conclusion
In CKD patients with no clinical or conventional echocardiographic evidence of significant CVD, GLS and aPWV independently predict the occurrence of MACE. If our findings are validated in larger prospective studies, GLS and aPWV may become a useful addition to the current risk stratification tools, aiming to prevent future cardiovascular events in this high-risk patient cohort.
References
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351(13):1296–1305
London GM, Parfrey PS (1997) Cardiac disease in chronic uremia: pathogenesis. Adv Ren Replace Ther 4(3):194–211
Rubin MF, Rosas SE, Chirinos JA, Townsend RR (2011) Surrogate markers of cardiovascular disease in CKD: what’s under the hood? Am J Kidney Dis 57(3):488–497
Poulikakos D, Ross L, Recio-Mayoral A, Cole D, Andoh J, Chitalia N, Sharma R, Carlos Kaski J, Banerjee D (2014) Left ventricular hypertrophy and endothelial dysfunction in chronic kidney disease. Eur Heart J Cardiovasc Imaging 15(1):56–61
Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort Study G (2012) Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23(10):1725–1734
Payne J, Sharma S, De Leon D, Lu JL, Alemu F, Balogun RA, Malakauskas SM, Kalantar-Zadeh K, Kovesdy CP (2012) Association of echocardiographic abnormalities with mortality in men with non-dialysis-dependent chronic kidney disease. Nephrol Dial Transplant 27(2):694–700
Chen SC, Su HM, Hung CC, Chang JM, Liu WC, Tsai JC, Lin MY, Hwang SJ, Chen HC (2011) Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol 6(12):2750–2758
Wu IW, Hung MJ, Chen YC, Hsu HJ, Cherng WJ, Chang CJ, Wu MS (2010) Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol 23(2):181–188
Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, Love TE, Aban IB, Shlipak MG (2007) Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol 99(3):393–398
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 12(3):167–205
Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA (2006) Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47(4):789–793
Kramann R, Erpenbeck J, Schneider RK, Rohl AB, Hein M, Brandenburg VM, van Diepen M, Dekker F, Marx N, Floege J, Becker M, Schlieper G (2014) Speckle tracking echocardiography detects uremic cardiomyopathy early and predicts cardiovascular mortality in ESRD. J Am Soc Nephrol 25(10):2351–2365
Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Leano R, Haluska BA, Stanton T (2014) The association between left ventricular global longitudinal strain, renal impairment and all-cause mortality. Nephrol Dial Transplant 29(6):1218–1225
Baumann M, Wassertheurer S, Suttmann Y, Burkhardt K, Heemann U (2014) Aortic pulse wave velocity predicts mortality in chronic kidney disease stages 2–4. J Hypertens 32(4):899–903
McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, Investigators A (2005) Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46(9):1753–1760
Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P, Nephrotest Study G (2011) Arterial remodeling associates with CKD progression. J Am Soc Nephrol 22(5):967–974
Chen SC, Chang JM, Liu WC, Tsai YC, Tsai JC, Hsu PC, Lin TH, Lin MY, Su HM, Hwang SJ, Chen HC (2011) Brachial-ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol 6(4):724–732
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130(6):461–470
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3):233–270
Devereux RB, Reichek N (1977) Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55(4):613–618
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent, Liege B, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17(12):1321–1360
Takemoto Y, Pellikka PA, Wang J, Modesto KM, Cauduro S, Belohlavek M, Seward JB, Thomson HL, Khandheria B, Abraham TP (2005) Analysis of the interaction between segmental relaxation patterns and global diastolic function by strain echocardiography. J Am Soc Echocardiogr 18(9):901–906
Ring M, Eriksson MJ, Zierath JR, Caidahl K (2014) Arterial stiffness estimation in healthy subjects: a validation of oscillometric (Arteriograph) and tonometric (SphygmoCor) techniques. Hypertens Res 37(11):999–1007
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T, Artery S, European Society of Hypertension Working Group on Vascular S, Function, European Network for Noninvasive Investigation of Large A (2012) Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30 (3):445–448
Sulemane S, Panoulas VF, Konstantinou K, Bratsas A, Tam FW, Brown EA, Nihoyannopoulos P (2016) Left ventricular twist mechanics and its relation with aortic stiffness in chronic kidney disease patients without overt cardiovascular disease. Cardiovasc Ultrasound 14:10
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100(21):1673–1680
Ersboll M, Valeur N, Mogensen UM, Andersen MJ, Moller JE, Hassager C, Sogaard P, Kober L (2012) Relationship between left ventricular longitudinal deformation and clinical heart failure during admission for acute myocardial infarction: a two-dimensional speckle-tracking study. J Am Soc Echocardiogr 25(12):1280–1289
Ternacle J, Berry M, Alonso E, Kloeckner M, Couetil JP, Rande JL, Gueret P, Monin JL, Lim P (2013) Incremental value of global longitudinal strain for predicting early outcome after cardiac surgery. Eur Heart J Cardiovasc Imaging 14(1):77–84
Cho GY, Marwick TH, Kim HS, Kim MK, Hong KS, Oh DJ (2009) Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol 54(7):618–624
Burns AT, La Gerche A, D’Hooge J, MacIsaac AI, Prior DL (2010) Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur J Echocardiogr 11(3):283–289
Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK (2008) Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging 1(3):366–376
Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C (2008) Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation 118(24):2571–2587
Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O (1999) Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 34(1):125–134
Weber KT, Brilla CG, Campbell SE, Zhou G, Matsubara L, Guarda E (1992) Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press 1(2):75–85
Stanton T, Leano R, Marwick TH (2009) Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2(5):356–364
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550
Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF (2008) Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J 29(10):1283–1289
Lumens J, Delhaas T, Arts T, Cowan BR, Young AA (2006) Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am J Physiol Heart Circ Physiol 291(4):H1573–H1579
Amundsen BH, Crosby J, Steen PA, Torp H, Slordahl SA, Stoylen A (2009) Regional myocardial long-axis strain and strain rate measured by different tissue Doppler and speckle tracking echocardiography methods: a comparison with tagged magnetic resonance imaging. Eur J Echocardiogr 10(2):229–237
Lai YH, Lo CI, Wu YJ, Hung CL, Yeh HI (2013) Cardiac Remodeling, Adaptations and Associated Myocardial Mechanics in Hypertensive Heart Diseases. Acta Cardiol Sin 29(1):64–70
van Huis M, Schoenmaker NJ, Groothoff JW, van der Lee JH, van Dyk M, Gewillig M, Koster L, Tanke R, Lilien M, Blom NA, Mertens L, Kuipers IM (2016) Impaired longitudinal deformation measured by speckle-tracking echocardiography in children with end-stage renal disease. Pediatr Nephrol 31(9):1499–1508
Takeuchi M, Borden WB, Nakai H, Nishikage T, Kokumai M, Nagakura T, Otani S, Lang RM (2007) Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: a study using two-dimensional speckle tracking imaging. Eur Heart J 28(22):2756–2762
Takeuchi M, Nishikage T, Nakai H, Kokumai M, Otani S, Lang RM (2007) The assessment of left ventricular twist in anterior wall myocardial infarction using two-dimensional speckle tracking imaging. J Am Soc Echocardiogr 20(1):36–44
Russel IK, Brouwer WP, Germans T, Knaapen P, Marcus JT, van der Velden J, Gotte MJ, van Rossum AC (2011) Increased left ventricular torsion in hypertrophic cardiomyopathy mutation carriers with normal wall thickness. J Cardiovasc Magn Reson 13:3
Fonseca CG, Dissanayake AM, Doughty RN, Whalley GA, Gamble GD, Cowan BR, Occleshaw CJ, Young AA (2004) Three-dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. Am J Cardiol 94(11):1391–1395
Hansen DE, Daughters GT 2nd, Alderman EL, Ingels NB Jr, Miller DC (1988) Torsional deformation of the left ventricular midwall in human hearts with intramyocardial markers: regional heterogeneity and sensitivity to the inotropic effects of abrupt rate changes. Circ Res 62(5):941–952
Panoulas VF, Sulemane S, Konstantinou K, Bratsas A, Elliott SJ, Dawson D, Frankel AH, Nihoyannopoulos P (2014) Early detection of subclinical left ventricular myocardial dysfunction in patients with chronic kidney disease. Eur Heart J Cardiovasc Imaging 16(5):539–548
de Bie MK, Ajmone Marsan N, Gaasbeek A, Bax JJ, Groeneveld M, Gabreels BA, Delgado V, Rabelink TJ, Schalij MJ, Jukema JW (2012) Left ventricular diastolic dysfunction in dialysis patients assessed by novel speckle tracking strain rate analysis: prevalence and determinants. Int. J Nephrol 2012:963504
Park JH, Marwick TH (2011) Use and limitations of E/e’ to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound 19(4):169–173
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102(15):1788–1794
Wang AY, Wang M, Lam CW, Chan IH, Zhang Y, Sanderson JE (2008) Left ventricular filling pressure by Doppler echocardiography in patients with end-stage renal disease. Hypertension 52(1):107–114
Kim MK, Kim B, Lee JY, Kim JS, Han BG, Choi SO, Yang JW (2013) Tissue Doppler-derived E/e’ ratio as a parameter for assessing diastolic heart failure and as a predictor of mortality in patients with chronic kidney disease. Korean J Intern Med 28(1):35–44
Chen SC, Chang JM, Tsai YC, Huang JC, Chen LI, Su HM, Hwang SJ, Chen HC (2013) Ratio of transmitral E-wave velocity to early diastole mitral annulus velocity with cardiovascular and renal outcomes in chronic kidney disease. Nephron Clin Pract 123(1–2):52–60
Briet M, Bozec E, Laurent S, Fassot C, London GM, Jacquot C, Froissart M, Houillier P, Boutouyrie P (2006) Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int 69(2):350–357
Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M, Nephro Test Study G (2012) Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension 60(6):1451–1457
Wang MC, Tsai WC, Chen JY, Huang JJ (2005) Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 45(3):494–501
Manovel A, Dawson D, Smith B, Nihoyannopoulos P (2010) Assessment of left ventricular function by different speckle-tracking software. Eur J Echocardiogr 11(5):417–421
Tucker PS, Scanlan AT, Dalbo VJ (2015) Chronic kidney disease influences multiple systems: describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev 2015:806358
Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J (2004) The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 140(1):9–17
Funding
S.S. received a doctoral research grant from Fundação para a Ciência e Tecnologia (FCT) under the grant number SFRH/BD/73646.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.S., V.P., A.B., J.G., E.B., and P.N. declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sulemane, S., Panoulas, V.F., Bratsas, A. et al. Subclinical markers of cardiovascular disease predict adverse outcomes in chronic kidney disease patients with normal left ventricular ejection fraction. Int J Cardiovasc Imaging 33, 687–698 (2017). https://doi.org/10.1007/s10554-016-1059-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-1059-x