Abstract
To develop more sensitive measures of impaired cardiac function in patients with pulmonary hypertension (PH), since detection of impaired right ventricular (RV) function is important in these patients. With the hypothesis that a change in septal function in patients with PH is associated with altered longitudinal and lateral function of both ventricles, as a compensatory mechanism, we quantified the contributions of these parameters to stroke volume (SV) in both ventricles using cardiac magnetic resonance (CMR). Seventeen patients (10 females) evaluated for PH underwent right heart catheterization (RHC) and CMR. CMR from 33 healthy adults (13 females) were used as controls. Left ventricular (LV) atrioventricular plane displacement (AVPD) and corresponding longitudinal contribution to LVSV was lower in patients (10.8 ± 3.2 mm and 51 ± 12 %) compared to controls (16.6 ± 1.9 mm and 59 ± 9 %, p < 0.0001 and p < 0.01, respectively). This decrease did not differ in patient with ejection fraction (EF) >50 % and <50 % (p = 0.5) and was compensated for by increased LV lateral contribution to LVSV in patients (49 ± 13 % vs. 37 ± 7 %, p = 0.001). Septal motion contributed less to LVSV in patients (5 ± 8 %) compared to controls (8 ± 4 %, p = 0.05). RV AVPD was lower in patients (12.0 ± 3.6 mm vs. 21.8 ± 2.2 mm, p < 0.0001) but longitudinal and lateral contribution to RVSV did not differ between patients (78 ± 17 % and 29 ± 16 %) and controls (79 ± 9 % and 31 ± 6 % p = 0.7 for both) explained by increased RV cross sectional area in patients. LV function is affected in patients with PH despite preserved global LV function. The decreased longitudinal contribution and increased lateral contribution to LVSV was not seen in the RV, contrary to previous findings in patients with volume loaded RVs.
Similar content being viewed by others
Introduction
Patients with pulmonary hypertension (PH) have a high mortality [1, 2]. Symptoms can be subtle and there is frequently a long delay to diagnosis [1]. When global right ventricular (RV) dysfunction occurs, the patient with PH is in a symptomatic and decompensated state with a low likelihood of being reversible [3]. Therefore, there is a need to find the early signs of impaired function to prevent deterioration of global function by early initiation of treatment in presymptomatic patients.
RV function and stroke volume (SV) are of prognostic value in these patients [2, 4–6]. SV is generated from different regional contributions of ventricular function; the longitudinal component caused by longitudinal shortening and atrioventricular plane displacement (AVPD) as well as the radial inward motion of the epicardial walls [7]. This radial component can be subdivided into the septal and lateral contributions to ventricular SV. We have previously quantified these contributions in healthy controls [7–10] and in patients with volume overloaded RV [11]. Septal function has been proposed to be a principal component of ventricular interdependence describing the coupling of the left ventricular (LV) and RV function [12–14]. Altered ventricular coupling is thought to be an explanation of the impaired regional LV function in PH patients despite preserved left ventricular ejection fraction (LVEF) [14–16].
Therefore, we hypothesized that a change in septal function in patients with PH is associated with altered longitudinal and lateral function of both the ventricles as a compensatory mechanism. To this end, we quantified the longitudinal, septal and lateral contributions to ventricular SVs using cardiac magnetic resonance imaging (CMR) providing a more detailed understanding of pumping physiology compared to global function in patients with PH.
Materials and methods
Seventeen adult patients (10 females) evaluated with right heart catheterization (RHC) and CMR for precapillary PH in Lund were included between 2003 and 2010. Adult patients with PH due to portal hypertension, human immunodeficiency virus infection, veno-occlusive disease, LV or congenital heart diseases were not included. Thirty-three healthy adult volunteers (13 females) were included as controls. The control group has been described in a previous study [11]. The regional ethical review board approved the study and written informed consent was obtained from all patients and healthy volunteers prior to CMR examination.
Right heart catheterization
Invasive measurements were obtained during RHC with a triple-lumen Swan-Ganz catheter in supine position and in local anesthesia. Pressures of pulmonary artery (PAP), right atrium, right ventricle and pulmonary artery wedge as well as cardiac output using thermodilution were measured. Precapillary PH was characterized by elevated mean pulmonary arterial pressure (mPAP) ≥25 mmHg and pulmonary artery wedge pressure ≤15 mmHg at normal or reduced cardiac output. Systemic pressure was available using a cuff and sphygmomanometer.
Cardiac magnetic resonance imaging
Image acquisition
All subjects underwent CMR imaging in the supine position and images were acquired during end-expiratory breath-hold covering the entire heart, including both ventricles and atria. A 1.5 Tesla magnetic resonance imaging scanner was used for all studies (Philips Achieva, Best, The Netherlands). Steady state free precession cine images were acquired in the short-axis plane. Imaging parameters were typically: ECG triggering with acquired temporal resolution of typically 47 ms reconstructed to 30 time phases per cardiac cycle, repetition time 3 ms, echo time 1.4 ms, flip angle 60°, slice thickness 6–8 mm with no slice gap. Breath-hold times were typically 15 s. The long axis images were acquired in the LV two chamber view, LV outflow tract view and four-chamber view.
Image analysis
CMR was analyzed by two experienced examiners with the software segment v1.9 (http://segment.heiberg.se) [17]. LVSV and RVSV and mass were obtained from a three-dimensional coverage of the ventricles in short-axis image stacks by delineating the epicardial and endocardial borders of both ventricles in all slices in end-diastole (ED) and end-systole (ES). SV was calculated by subtracting ES volume from the ED volume. Mass was calculated as the myocardial volume times muscle density (1.05 g/ml).
Quantification of longitudinal contribution to stroke volume, SVlong%
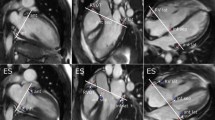
The AVPD was measured in three long-axis views by subtracting the AV plane position in ES from that in ED, as previously described [7, 8] (Fig. 1). In short, eight points were marked in each timeframe; three points in the four-chamber view (RV free wall as well as LV inferoseptal and anterolateral), three points in the three-chamber view (RV outflow tract as well as LV anteroseptal and inferolateral point) and two points in the two-chamber view (LV anterior and inferior). The RV AVPD was measured in the four-chamber RV-free wall, the RV outflow tract seen in the three-chamber view and from the mean of the two septal points in the four-chamber and three-chamber view. Using the mean of the septal points creates a simplified RV triangle for calculating RV AVPD and the method has been validated for calculation of longitudinal contribution to RVSV [7]. LV AVPD was measured in six points, two from each of the two-, three- and four-chamber views. The epicardial delineation from short-axis was used for calculating the epicardial areas in ED. Since the mean AVPD was larger than the slice thickness, the mean short-axis area was generated from the three largest areas in RV and two largest areas in LV encompassed by the AVPD [7]. The longitudinal contribution to SV was calculated as AVPD multiplied with mean short-axis epicardial area of RV and LV respectively. The resulting volumes from each ventricle was expressed as a percentage (SVlong%) of the SV from each ventricle (RVSVlong% and LVSVlong%) [7, 8].
Atrioventricular plane displacement in a heathy adult (top) and a patient with pulmonary hypertension (bottom). The atrioventricular plane (AVP) and displacement (AVPD) is demonstrated in four-chamber views in end-diastole (ED, a, c, full line) and in end-systole (ES, b, d, dashed line). The volume derived from the AVPD is the area between the full and dashed outline of the AVPD. The dashed boxes indicate the basal short-axis slices used when calculating the longitudinal contribution of stroke volume by multiplying the short-axis area with AVPD. Note that the AVPD in both the right and left ventricle is lower in the patient compared to the control
Quantification of septal and lateral contribution to stroke volume, SVsept% and SVlat%
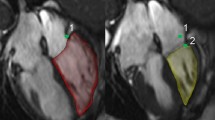
The RV insertion points to the LV in ED and ES were used to define the extent of the ventricular septum. The epicardial contours in ED were copied to the corresponding images in ES. The three-dimensional volume between the ED and ES epicardial contours were computed.
The septal volume was defined as the volume generated by the septal movement between the RV insertion points. At the base and apex, only images with ventricular septum present in both ED and ES were used (Fig. 2). The septal contribution to SV (SVsept%) is the septal volume in percentage of the total LVSV. Positive value of SVsept% implies the septum moves towards the left side in systole and therefore contributes to the LVSV. On the other hand, with a negative value of SVsept% the septum moves to the right side in systole and contributes negatively to the LVSV (Fig. 2) and correspondingly positive to RVSV.
Lateral and septal movement in a healthy control (top) and a patient with pulmonary hypertension (bottom) demonstrated in the short axis. The lateral and septal movement is demonstrated in short axis views in end-diastole (ED, a, c, full line) and in end-systole (ES, b, d, dashed line). The right ventricular insertion points are marked with double circles. The faintly colored areas are the septal contribution to stroke volume. The septum is moving to the left in the healthy control in systole, and in this patient the septum is moving both to the left and right in systole, while bulging into the left ventricle. The volume derived from the lateral movement is the area between the full and dashed epicardial delineations without the septum. Note that in the patient, the left ventricular lateral contribution is larger, the septum is flattened and the volumetric measures are RV EDV 206 ml, RVEF 34 %, LV EDV 102 ml, LVEF 55 %
The lateral volume was defined as the three-dimensional volume between the epicardial delineations in ED and ES excluding the volume between the septal RV insertions to the LV. The lateral contribution to SV (SVlat%) is the lateral volume in percentage of SV from each corresponding ventricles.
We have formerly studied the interobserver variability of the method in our group and we have shown good agreement for LV and RV volumes as well as for LV and RV AVPD [9, 11]. LVSV measured from aortic flow has been compared to LVSV from planimetry with a small bias of 5.4 ± 7.8 % [9] and the interobserver variability for LV AVPD was −0.7 ± 1.3 mm and for RV AVPD 1.1 ± 1.3 mm [9]. Therefore, we used a second observer for quality assessment.
Statistical analysis
All statistical analysis was performed using Graphpad Prism v 5.02. Continuous variables were presented as mean ± SD. Pearson’s correlation was used to examine the relationship between pulmonary pressure and the longitudinal, septal and lateral contribution to SV as well as the relationship between pressure and ventricular mass. Mann–Whitney test was used to test if variables differed between the groups. Results with a two-sided p value of <0.05 were considered statistically significant.
Results
Subject characteristics
Age, body surface area (BSA), ventricular volumes, ventricular mass, EF for the study groups as well as RHC measurements for the patients with PH are presented in Table 1 and clinical characteristics in Table 2. Patients with PH had smaller left ventricles, larger right ventricles and lower SV compared to controls (Table 1).
Longitudinal contribution to stroke volume, SVlong%
The AVPD in the PH population was lower (LV 10.8 ± 3.2 mm and RV 12.0 ± 3.6 mm) than in controls (LV 16.6 ± 1.9 mm and RV 21.8 ± 2.2 mm, p < 0.0001 for both) (Fig. 3). However, RVSVlong% was not different in PH patients from healthy subjects (78 ± 17 % vs. 79 ± 9 %, p = 0.7 (Table 3; Fig. 4). This was explained by larger RV area in patients and lower SVs compared to controls (mean RV area was 40.6 ± 8.4 cm2 in patients with PH and 32.4 ± 4.4 cm2 in healthy controls; p = 0.0006) (Table 1). Conversely, LVSVlong% was significantly smaller in the PH population compared to healthy adults (51 ± 12 % vs. 59 ± 9 %, p = 0.008 (Table 3; Fig. 4). There was no correlation between LVSVlong% and LVEF (p = 0.1) and there was no difference in LVSVlong% for the patients with LVEF more than and equal to 50 % and those with less than 50 % (p = 0.5).
Neither SVlong% (Fig. 5) nor AVPD, on either side, correlated with systolic pulmonary arterial pressure (sPAP), pulmonary vascular resistance (PVR) or right ventricular mass (RVM) in patients with PH (p = NS).
Linear correlation analysis of longitudinal, septal and lateral contribution to stroke volume compared to systolic pulmonary arterial pressure (sPAP) in patients with pulmonary hypertension. a Correlation to right ventricular (RV) longitudinal contribution (RVSVlong%). b Correlation to left ventricular contribution to stroke volume (LVSVlong%). c Correlation to septal contribution to SV (SVsept%). Positive values of septal volume (%) mean that the septum moves to the left in systole and a negative value that it moves to the right. d Correlation to RV lateral contribution to stroke volume (RVSVlat%)
Lateral contribution to stroke volume, SVlat%
The RVSVlat% did not differ between controls and PH patients (31 ± 6 % vs. 29 ± 16 %, p = 0.7) whereas LVSVlat% was significantly larger in the PH population compared to the healthy subjects (49 ± 13 % vs. 37 ± 7 %, p = 0.001). There was no correlation between SVlat% and sPAP, PVR or RVM, on either side (p = NS for all).
Septal contribution to stroke volume, SVsept%
In PH patients, there was a tendency to a lower SVsept%, but this difference was not significant, (5 ± 8 %, p = 0.05) compared to healthy controls (8 ± 4 %) (Table 3; Fig. 4). There was no correlation between SVsept% and sPAP (Fig. 5), PVR or RVM (p = NS).
Interventricular dependency
In patients with PH, SVsept% correlated positively with RVSVlong% (r = 0.528, p = 0.0003) and RVSVlat% correlated inversely with RVSVlong% (r = −0.792, p = 0.0002) (Fig. 6a, c). In controls, SVsept% showed a correlation to RVSVlong% (r = 0.424, p = 0.01), but there was no correlation between RVSVlat% and RVSVlong% (p = NS) (Fig. 6b, d).
Discussion
This study has quantified the longitudinal, septal and lateral contribution to SV in patients with PH. We found decreased longitudinal contribution to SV in the left ventricle and unchanged contribution in the right ventricle. The LV lateral contribution to SV was increased, possibly as a compensatory mechanism to the decreased longitudinal function. Septal contribution to LVSV showed a tendency to be lower in patients compared to healthy controls. These changes in patients with a pressure loaded RV differ from previous findings in patients with volume loaded RV. Therefore, volume and pressure load of the right side of the heart yield different pumping physiology in both the right as well as the left ventricle.
The results indicate that in the pressure loaded RV, there is not only an interventricular dependency, but also an intraventricular compensatory mechanism, that is changed compared to healthy controls. We might therefore be able to detect a more subtle deterioration of the RV function and this may be used to follow patients on an individual basis.
This study has hereby shown that LV regional pumping modes are affected in patients with PH, even when global systolic LV function is unaffected.
Relation to earlier studies
With the principle of the muscle mass being constant throughout the heart cycle, both epicardial and endocardial contours have been validated to be applicable for SV calculations using CMR [7]. Since the outer contour of the whole heart changes minimally through the heart cycle, the lateral contribution to SV has been distinguished by the measurement of the epicardial and not the endocardial volume change from CMR [7, 10]. The wall thickening from the longitudinal displacement will affect the measurement of the lateral contribution, if measured from the endocardium wall or from the midwall. Endocardial change is therefore not equal to lateral function, but to a high extent a result of the AVPD and hence thickening of the wall. The myocardium has to thicken in the shorter ventricle, even without any radial squeezing movement [7, 10]. In other words, even with no radial contribution to SV, there will still be an inward motion of the endocardium. This explains why we use the epicardial contour of the ventricle to calculate the longitudinal and lateral contributions to SV [7, 10].
The AVPD, and thus longitudinal function, were in absolute numbers smaller in PH patients on both the right and left side compared to the control group. As previously shown using speckle-tracking strain from echocardiography, deterioration of the longitudinal RV function is associated with poor outcome [18]. Similarly, a decrease in tricuspid annular plane systolic excursion has been shown with echocardiography in PH patients as having prognostic value [19, 20]. Our findings are in concordance with these previous studies and also a recent study by Swift et al. on longitudinal and transverse RV function in PH with CMR [21]. Swift et al. showed, that tricuspid annular plane systolic excursion was lower in patients with elevated pulmonary pressure [21]. When the right ventricle dilates and the septum protrudes into the LV cavity, the LV cavity becomes small and compressed by the large right ventricle [22]. The RV gets a more spherical appearance, resembling that of a normal left ventricle and the longitudinal muscle fibers change direction to a more circumferential direction [23].
Interestingly, the decreased absolute AV-plane displacement in PH patients did not cause a decrease in longitudinal contribution to RVSV (RVSVlong%), as would have been expected. This was due to the increased RV area caused by RV dilatation and the decreased SV. Similar mechanisms have been shown in patients with LV heart failure, where AVPD is decreased and LV short-axis area increased, thereby preserving longitudinal pump function [8].
LVSVlong% was lower in the PH population compared to healthy subjects, and an increased LVSVlat% compensated for the decreased LV AVPD. This is in line with the reduced longitudinal speckle-tracking strain values with echocardiography in PH patients with normal systolic function [12].
Septal movement contributed mainly to LVSV in systole (positive value of SVsept%) in both PH patients and controls, though with a large range in the PH patients. Where the septum is convex in the healthy subjects, it is flattened or even concave into the LV in the PH patients, giving the RV a more spherical appearance, resembling a left ventricle [21, 23]. These studies by Swift et al. and Grapsa et al. [21, 23] as well as the study by Mauritz et al. [24] have reported that changes in regional diameter, sphericity index from diameter or other two-dimensional regional measurements carry prognostic information of poor outcome. They suggest that the contributions to transverse and longitudinal motion using three-dimensional imaging may improve accuracy. Structures can translate out of plane, giving rise to risk of false shortening or lengthening, which is a challenge to take into account for in two-dimensional images. Swift et al. proposed that longitudinal and transverse motion in one plane may therefore not fully represent the relative contributions to RV function [21]. In our study, we have used a three-dimensional approach to calculate regional contributions to SV and do not approximate regional function from two-dimensional image planes.
Comparison between pressure and volume loaded right ventricles
In patients with pulmonary regurgitation and volume loaded right ventricles, the septum moves paradoxically to the right in systole contributing to RVSV [25]. In diastole the septal shape is flattened [11, 13, 26]. Patients with PH, on the other hand, have pressure loaded right ventricles resulting in RV hypertrophy, paradoxical septal movement and a “flattening” of the septal shape in systole [23, 25].
In our population of PH patients, the septum moved mainly towards the left ventricle in systole, though interestingly, with an impaired LVSVlong% and there is increased LVSVlat%. The septal contribution differs from patients with volume loaded RV such as patients with pulmonary regurgitation or atrial septal defects where the septum moves towards the right side in systole [11, 13, 25, 26]. The movement of septum towards the left in our study is in concordance with earlier studies with pressure loaded RV [16, 27–29]. SVlong% was decreased on the left side in patients with PH compared to controls, but did not differ on the right side between patients and controls (Table 3; Fig. 4). This result also differs from the volume loaded RV due to pulmonary regurgitation where the LVSVlong% is mainly unchanged [11] but the contribution of the LV lateral wall even more increased (58 ± 13 %) to compensate for the paradoxical septal movement [11]. The RVSVlong% is on the other hand lower in volume loaded RV, due to pulmonary regurgitation, compared to healthy subjects and is compensated with an increased RVSVlat%.
There could be several mechanisms to explain these differences. One explanation could be that the LV is volume depleted in PH patients and cannot be “filled out”, especially when the pressure is severely raised on the right side [30]. Several studies have shown that the LV function is impaired despite preserved LVEF in PH patients [12, 15, 16, 31, 32] and some studies have suggested that this could be caused by impaired LV filling rather than a “true” diastolic dysfunction [15, 30, 32]. The contractile function and the cross sectional area of LV cardiomyocytes is substantially reduced in PH patients, which would support a true regional dysfunction [33]. In that relation, the lack of prestretch of the LV due to volume depletion could be a contributing factor to the contractile function of the myocytes. Also, LV afterload can have an impact on the LV longitudinal and diastolic function, yet in our material the systemic blood pressures did not differ substantially between the groups. The decreased cardiac index and preserved LVEF in our study support the hypothesis of LV volume depletion. Of note, the cardiac index was lower in the PH patients compared to the previously studied patients with volume loaded RVs [11]. Another reason could be the RV hypertrophy in patients with PH [right ventricular mass index (RVMI), 32 ± 16 g/m2] compared to healthy subjects and to patients with volume loaded RVs (RVMI, 22 ± 15 g/m2, previously unpublished data) [11]. The RV hypertrophy in our study is in concordance with earlier studies [6, 34]. This suggests, that the remodeling of the RV in PH patients may differ compared to patients with volume loaded RVs. We have included a regression analysis between RV ventricular mass and the regional contributions to SV on both sides. We found no correlations, neither linear nor non-linear but this needs to be further tested in larger patient cohorts.
RV intraventricular compensation
In the PH patients the range of both RVSVlong% and SVsept% are wide and encompasses extremes such as e.g. RVSVlong% of 114 % compensating for a SVsept% of 19 % (septal movement towards the left) in one patient (Table 3). When the septum contributes the most to the LV, the septum is bulging concave into the left ventricle in systole in patients with PH. To compensate for the negative contribution to RVSV, RVSVlong% can be even greater than the total RVSV. On the other hand, when RVSVlong% decreases in PH patients, RVSVlat% increases, and exceeds the lateral contribution in healthy controls (Fig. 6). As such, when one part of the RV function is altered another takes over. This could indicate that in the pressure loaded RV there is not only an interventricular dependency but also an intricate intraventricular compensatory mechanism. Since this shift can be quantified instead of approximated, it is now possible to follow patients on an individual basis and thus we might be able to detect more subtle deterioration of the RV function. If there are prognostic implications of these measures of ventricular function remains to be investigated.
Limitations
The genesis of PH is, in our study, somewhat heterogeneous with a variation of different levels of pulmonary arterial pressure, RV hypertrophy and RVSV. Yet all patients had precapillary PH. It has been suggested that the genesis of PH may lead to diverse remodeling of RV [27, 35]. Our study was not designed to accommodate a differentiation of these factors.
The age difference between controls and patients may be considered a limitation in the study. In a posthoc analysis of the healthy control group, we found an inverse correlation between RV AVPD and age (r = −0.39, p = 0.02), but no correlation between LV AVPD and age (p = 0.15). On the other hand, when correlating the different regional contributions to SV to age, there was no significance in any of the measures. Furthermore, we found no difference in regional contributions in healthy controls >50 years compared to those <50 years. Therefore, age does not appear to influence the results of the regional contribution to SV. The sample size of this proof of concept study is small and inclusion was retrospective. Therefore, the new findings of this study need to be tested in larger patient cohorts to show the relationship with clinical outcome measures.
Conclusion
The longitudinal, septal and lateral contributions to ventricular stroke SVs can be quantified by using CMR. LV function is affected in patients with PH even when global systolic LV function is preserved. The changes with decreased longitudinal contribution to LVSV and increased lateral contribution to LVSV are not seen in the RV, contrary to previous findings in patients with volume loaded RVs. Thus, volume and pressure load of the right side of the heart yield different pumping physiology in the right as well as the left ventricle. This study was a proof of concept study in a retrospective patient cohort with PH. A longitudinal follow up with prognostic markers would be of interest in the future to determine if these measures of pumping physiology may detect subtle deterioration of the LV function that can be used to follow patients on an individual basis.
Abbreviations
- AVPD:
-
Atrioventricular plane displacement
- CMR:
-
Cardiac magnetic resonance imaging
- ED:
-
End-diastole
- EF:
-
Ejection fraction
- ES:
-
End-systole
- LV:
-
Left ventricular
- LVM:
-
Left ventricular mass
- mPAP:
-
Mean pulmonary arterial pressure
- PH:
-
Pulmonary hypertension
- PVR:
-
Pulmonary vascular resistance
- RHC:
-
Right heart catheterization
- RV:
-
Right ventricular
- RVM:
-
Right ventricular mass
- sPAP:
-
Systolic pulmonary arterial pressure
- SV:
-
Stroke volume
- SVlat% :
-
Lateral contribution to stroke volume
- SVlong% :
-
Longitudinal contribution to stroke volume
- SVsept% :
-
Septal contribution to stroke volume
References
Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S (2007) An epidemiological study of pulmonary arterial hypertension. Eur Respir J 30:104–109
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A et al. (2016) 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37(1):67–119
Rich S (1988) Primary pulmonary hypertension. Prog Cardiovasc Dis 31:205–238
Chin KM, Kingman M, de Lemos JA, Warner JJ, Reimold S, Peshock R et al (2008) Changes in right ventricular structure and function assessed using cardiac magnetic resonance imaging in bosentan-treated patients with pulmonary arterial hypertension. Am J Cardiol 101:1669–1672
D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM et al (1991) Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115:343–349
van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD et al (2007) Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 28:1250–1257
Carlsson M, Ugander M, Heiberg E, Arheden H (2007) The quantitative relationship between longitudinal and radial function in left, right, and total heart pumping in humans. Am J Physiol 293:H636–H644
Carlsson M, Ugander M, Mosen H, Buhre T, Arheden H (2007) Atrioventricular plane displacement is the major contributor to left ventricular pumping in healthy adults, athletes, and patients with dilated cardiomyopathy. Am J Physiol 292:H1452–H1459
Steding-Ehrenborg K, Carlsson M, Stephensen S, Arheden H (2013) Atrial aspiration from pulmonary and caval veins is caused by ventricular contraction and secures 70 % of the total stroke volume independent of resting heart rate and heart size. Clin Physiol Funct Imaging 33:233–240
Ugander M, Carlsson M, Arheden H (2010) Short-axis epicardial volume change is a measure of cardiac left ventricular short-axis function, which is independent of myocardial wall thickness. Am J Physiol 298:H530–H535
Stephensen SS, Steding-Ehrenborg K, Arheden H, Heiberg E, Carlsson M (2014) The relationship between longitudinal, lateral and septal contribution to stroke volume in patients with pulmonary regurgitation and healthy volunteers. Am J Physiol 306(6):H895–H903
Hardegree EL, Sachdev A, Fenstad ER, Villarraga HR, Frantz RP, McGoon MD et al (2013) Impaired left ventricular mechanics in pulmonary arterial hypertension: identification of a cohort at high risk. Circ Heart Fail 6:748–755
Weyman AE, Wann S, Feigenbaum H, Dillon JC (1976) Mechanism of abnormal septal motion in patients with right ventricular volume overload: a cross-sectional echocardiographic study. Circulation 54:179–186
Lazar JM, Flores AR, Grandis DJ, Orie JE, Schulman DS (1993) Effects of chronic right ventricular pressure overload on left ventricular diastolic function. Am J Cardiol 72:1179–1182
Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC et al (2001) Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest 119:1761–1765
Alpert JS (2001) The effect of right ventricular dysfunction on left ventricular form and function. Chest 119:1632–1633
Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H (2010) Design and validation of segment—freely available software for cardiovascular image analysis. BMC Med Imaging 10:1
Hardegree EL, Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Kushwaha SS et al (2013) Role of serial quantitative assessment of right ventricular function by strain in pulmonary arterial hypertension. Am J Cardiol 111:143–148
Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA et al (2006) Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 174:1034–1041
Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W et al (2002) Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 39:1214–1219
Swift AJ, Rajaram S, Capener D, Elliot C, Condliffe R, Wild JM et al (2015) Longitudinal and transverse right ventricular function in pulmonary hypertension: cardiovascular magnetic resonance imaging study from the ASPIRE registry. Pulm Circ 5:557–564
Champion HC, Michelakis ED, Hassoun PM (2009) Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation 120:992–1007
Grapsa J, Gibbs JS, Cabrita IZ, Watson GF, Pavlopoulos H, Dawson D et al (2012) The association of clinical outcome with right atrial and ventricular remodelling in patients with pulmonary arterial hypertension: study with real-time three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 13:666–672
Mauritz GJ, Kind T, Marcus JT, Bogaard HJ, van de Veerdonk M, Postmus PE et al (2012) Progressive changes in right ventricular geometric shortening and long-term survival in pulmonary arterial hypertension. Chest 141:935–943
Kaul S (1986) The interventricular septum in health and disease. Am Heart J 112:568–581
Walker RE, Moran AM, Gauvreau K, Colan SD (2004) Evidence of adverse ventricular interdependence in patients with atrial septal defects. Am J Cardiol 93:(1374–1377), A1376
Grapsa J, Gibbs JS, Dawson D, Watson G, Patni R, Athanasiou T et al (2012) Morphologic and functional remodeling of the right ventricle in pulmonary hypertension by real time three dimensional echocardiography. Am J Cardiol 109:906–913
Lima JA, Guzman PA, Yin FC, Brawley RK, Humphrey L, Traill TA et al (1986) Septal geometry in the unloaded living human heart. Circulation 74:463–468
Morikawa T, Murata M, Okuda S, Tsuruta H, Iwanaga S, Satoh T et al (2011) Quantitative analysis of right ventricular function in patients with pulmonary hypertension using three-dimensional echocardiography and a two-dimensional summation method compared to magnetic resonance imaging. Am J Cardiol 107:484–489
Hsia HH, Haddad F (2012) Pulmonary hypertension: a stage for ventricular interdependence? J Am Coll Cardiol 59:2203–2205
Eysmann SB, Palevsky HI, Reichek N, Hackney K, Douglas PS (1989) Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation 80:353–360
Gurudevan SV, Malouf PJ, Auger WR, Waltman TJ, Madani M, Raisinghani AB et al (2007) Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol 49:1334–1339
Manders E, Bogaard HJ, Handoko ML, van de Veerdonk MC, Keogh A, Westerhof N et al (2014) Contractile dysfunction of left ventricular cardiomyocytes in patients with pulmonary arterial hypertension. J Am Coll Cardiol 64:28–37
Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A et al (2009) Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 54:S55–S66
Hesselstrand R, Scheja A, Wuttge DM, Arheden H, Ugander M (2011) Enlarged right-sided dimensions and fibrosis of the right ventricular insertion point on cardiovascular magnetic resonance imaging is seen early in patients with pulmonary arterial hypertension associated with connective tissue disease. Scand J Rheumatol 40:133–138
Acknowledgments
Special gratitude goes to Ann-Helen Arvidsson and Christel Carlander for the assistance in image acquisition and patient data management.
Funding
This study was funded by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Medical Faculty of Lund University, and the Region of Skåne.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/),which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ostenfeld, E., Stephensen, S.S., Steding-Ehrenborg, K. et al. Regional contribution to ventricular stroke volume is affected on the left side, but not on the right in patients with pulmonary hypertension. Int J Cardiovasc Imaging 32, 1243–1253 (2016). https://doi.org/10.1007/s10554-016-0898-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0898-9