Abstract
Knowledge about potential differences in infarct tissue characteristics between patients with prior life-threatening ventricular arrhythmia versus patients receiving prophylactic implantable cardioverter-defibrillator (ICD) might help to improve the current risk stratification in myocardial infarction (MI) patients who are considered for ICD implantation. In a consecutive series of (ICD) recipients for primary and secondary prevention following MI, we used contrast-enhanced (CE) cardiovascular magnetic resonance (CMR) imaging to evaluate differences in infarct tissue characteristics. Cine-CMR measurements included left ventricular end-diastolic and end-systolic volumes (EDV, ESV), left ventricular ejection fraction (LVEF), wall motion score index (WMSI), and mass. CE-CMR images were analyzed for core, peri, and total infarct size, infarct localization (according to coronary artery territory), and transmural extent. In this study, 95 ICD recipients were included. In the primary prevention group (n = 66), LVEF was lower (23 ± 9 % vs. 31 ± 14 %; P < 0.01), ESV and WMSI were higher (223 ± 75 ml vs. 184 ± 97 ml, P = 0.04, and 1.89 ± 0.52 vs. 1.47 ± 0.68; P < 0.01), and anterior infarct localization was more frequent (P = 0.02) than in the secondary prevention group (n = 29). There were no differences in infarct tissue characteristics between patients treated for primary versus secondary prevention (P > 0.6 for all). During 21 ± 9 months of follow-up, 3 (5 %) patients in the primary prevention group and 9 (31 %) in the secondary prevention group experienced appropriate ICD therapy for treatment of ventricular arrhythmia (P < 0.01). There was no difference in infarct tissue characteristics between recipients of ICD for primary versus secondary prevention, while the secondary prevention group showed a higher frequency of applied ICD therapy for ventricular arrhythmia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ventricular arrhythmia (VA) is a major cause of sudden cardiac death (SCD) in patients with prior myocardial infarction (MI) [1]. Several randomized trials have shown a beneficial effect of implantable cardioverter-defibrillator (ICD) therapy among MI patients with prior life-threatening VA (secondary prevention) [2–4]. In the setting of prophylactic ICD therapy (primary prevention), left ventricular ejection fraction (LVEF) below 35 % is considered an indication for ICD implantation. However, <25 % of these ICD recipients actually experience a life-threatening VA requiring shock therapy during median follow-up of 45.5 months [5]. Current guidelines consider a low LVEF post-MI as the most important criterion to determine a patient’s eligibility. Therefore, these guidelines appear to be suboptimal [1]. Better risk stratification is warranted to reduce the number of unnecessary device implantations, especially in the setting of primary prevention [3, 5–7].
Cardiovascular magnetic resonance (CMR) imaging in combination with the contrast-enhancement (CE) technique allows the accurate assessment of LV geometry and function as well as tissue characteristics. This permits accurate assessment of size, heterogeneity, and transmurality of the myocardial scar [8, 9]. Infarct tissue characteristics (e.g. localization, heterogeneity) [10–13] are considered potential predictors of life-threatening VAs, and could play a role in risk stratification before ICD implantation [8, 9, 14].
Previous studies demonstrated a higher occurrence of VA (and thus a higher incidence of appropriate ICD therapy) in ICD recipients for secondary prevention compared to patients who received an ICD in the setting of primary prevention [2, 15–18]. Insight into potential differences in infarct tissue characteristics between ICD recipients for primary versus secondary prevention may potentially help to improve the current practice of risk stratification in MI patients considered for ICD implantation, specifically in the primary prevention group.
Therefore, in a consecutive series of ICD recipients for primary and secondary prevention following MI, we used CE-CMR to evaluate potential differences in infarct tissue characteristics.
Methods
Patient population
The study was conducted at Medisch Spectrum Twente, Enschede, the Netherlands. A consecutive series of patients with prior MI, who received an ICD for primary or secondary prevention following current guidelines of the Dutch (NVVC) and European society of Cardiology (ESC) in which the LVEF was determined based on echocardiographic findings, was assessed. The referring physicians had no access to the CMR report before defining therapeutic management. Prior to ICD implantation, these patients were referred for CMR to assess left ventricular (LV) dimension and function, and after intravenous injection of gadolinium, characterization of the infarcted tissue. According to current guidelines, the patients who received ICD treatment for primary prevention had an indication based on a LVEF ≤ 35 % (majority of patients) or the presence of spontaneous ventricular tachycardia, even with a somewhat more preserved LV function. Patients were only included in the study if the MI occurred at least 1 month prior to CMR (according to the definition of a healed MI [19] and a positive infarct pattern on CE imaging was found. The study was approved by the local ethics committee and informed consent was obtained.
As the secondary prevention group (dissimilar to the primary prevention group) was not selected based on a particularly low ejection fraction, the mean LVEF of this group may be expected to be higher. To correct for this potential confounder, ICD recipients from both groups with a LVEF ≤ 35 % were separately compared. A comparable subgroup analysis in ICD recipients with a LVEF > 35 % was not performed as the limited number of patients did not permit a meaningful analysis.
CMR data acquisition
CMR examination was performed on a 1.5-T whole body scanner (Achieva scan, Philips Medical System, Best, the Netherlands) using commercially available software. For signal-reception a five-element cardiac synergy coil was used. Electrocardiogram triggering was performed with a vector-electrocardiogram set-up. Subjects were examined in the supine position. Cine (morphologic) images in the cardiac short-axis, four-chamber, three-chamber, two-chamber long-axis, and LV outflow tract views were acquired by using fast field echo cine images. (Slice thickness 8.0 mm, repetition time 3.4 ms; echo time 1.7 ms; flip angle 60°; matrix 256 × 256).
Myocardial scar was assessed on CE multislice short- axis, long-axis and four-chamber views, obtained 10 min after intravenous bolus injection of 0.2 mmol gadolinium/kg body weight (Shering AG, Berlin, Germany). A three-dimensional Turbo Field Echo-inversion recovery T1-weighted sequence was used with the following parameters: repetition time 4.0 ms; echo time 1.3 ms; flip angle 15°; inversion time individually optimized to null myocardial signal (usually between 180 and 250 ms); matrix 157; and slice thickness 10 mm.
CMR data analysis and definitions
CMR data were analyzed on a workstation using dedicated software (Philips MR workspace, Release 2.5.3.0; the Netherlands). Analysis was performed by reviewers blinded to clinical information.
LV geometry and function
Left ventricular end-diastolic and end-systolic volumes (EDV and ESV; ml), left ventricular ejection fraction (LVEF; %), and end-diastolic wall mass (EDWM; g) were calculated from contiguous short-axis loops by segmentation of endocardial and epicardial borders on each frame. Body surface area adjusted EDV (EDVi), ESV (ESVi) and EDWM (EDWMi) were also calculated.
The left ventricular wall regions were further divided into 17 segments according to a standardized myocardial segmentation model [20]. Normal wall motion was assigned a score 0, hypokinesia 1, severe hypokinesia 2, akinesia 3, and dyskinesia 4. The wall motion score index (WMSI) was calculated by dividing the sum of scores in each segment by the total number of observed segments.
Infarct tissue characteristics
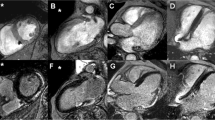
Infarcted myocardium was defined as the zone of hyperenhancement on the CE images, in contrast with the dark-gray signal of normal myocardium (Fig. 1). Infarct size was quantified by a semi-automatic thresholding technique with the full width at half maximum approach as previously validated [21]. After outlining the myocardial segment containing the region with high signal intensity, the maximum signal intensity region was determined. Scar was divided into an infarct core zone and a heterogeneous zone (i.e. peri-infarct zone). Infarct core was then defined as myocardium with a signal intensity ≥50 % of the maximal signal intensity. The heterogeneous zone was defined as myocardium with a signal intensity between ≥35 and <50 % of maximal signal intensity. Total scar was defined as the sum of infarct core plus heterogenous zone.
Scar tissue characteristics were further quantified according to location by use of a 17 segmental model [20]. Each segment was scored as follows: a scar score of 0 was defined normal, 1 as 1–25 % scar, 2 as 26–50 % scar, 3 as 51–75 % scar, and 4 as 76–100 % scar of the segmental area.
The transmural extent of myocardial scar was defined as the number of segments with a scar score 3 or 4 [22]. In addition, a segmental regional scar score was calculated in order to relate scar size to the territories of the three major coronary arteries as previously described in detail [20].
Follow up and definition of events
Follow-up was performed by our outpatient clinic, including registration of the occurrence of events and survival status. Regular device interrogation was scheduled every 3–6 months. In case of any experienced event, an additional device interrogation was performed. Device therapy was classified as appropriate or inappropriate. Appropriate ICD therapy was defined as anti-tachycardia pacing and/or appropriate shock in response to ventricular tachycardia or ventricular fibrillation. For the purpose of this study, only appropriate device therapies were considered as arrhythmic events. Mortality was reported and causes of death were scored as follows: (1) myocardial infarction, (2) heart failure, (3) cerebrovascular accident, (4) carcinoma, or (5) other causes of death.
A major cardiovascular event (MACE) was defined as appropriate ICD therapy and/or death.
Statistical analysis
Continuous variables had a normal distribution and were expressed as mean ± SD. Categorical data were expressed as frequencies and percentages. To compare the primary and secondary prevention groups, Student’s t test and Mann–Whitney U test were used to compare continuous variables, and Chi-square test and Fisher exact test were used to compare categorical variables. A survival analysis was performed to investigate if the association between infarct tissue characteristics with MACE is different among groups (ICD for primary preventions vs. ICD for secondary prevention). A P value < 0.05 was considered statistically significant.
Results
Study patients
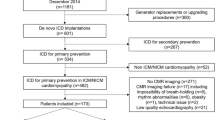
In this study, 95 patients (64 ± 10 years old; 79 men) with a median of 141 (1–434) months after MI were examined. A total of 66 patients received an ICD for primary prevention and 29 patients for secondary prevention. Indication for secondary prevention by ICD implantation was (1) SCD in 14 patients (48 %) or (2) VT episodes in 15 patients (52 %). Time between events and ICD implantation was on average 2 weeks (median). (25th percentile 1 week, 50th percentile 2 weeks, 75th percentile weeks). In general, ICD implantation was performed shortly after CE-CMR assessment; the average (median) within 1 week, the 75th percentile within 1 week and the 90th percentile within 5.4 weeks. Demographics and baseline characteristics did not differ between groups except for diuretic usage (80 % vs. 45 %; P < 0.01). Patient demographics are presented in Table 1, which also shows a subgroup of patients with LVEF ≤ 35 %.
CMR results
LV geometry and function
In the primary prevention group, LVEF (23 ± 9 % vs. 31 ± 14 %; P < 0.01) was significantly lower while ESVi (113 ± 39 ml vs. 91 ± 49 ml; P = 0.03) and WMSI (1.89 ± 0.52 vs. 1.47 ± 0.68; P < 0.01) were significantly higher than in the secondary prevention group (Table 2).
Infarct characteristics
There were no significant differences between size of the infarct core (12 ± 7 % vs. 11 ± 9 %; P = 0.62), size of the peri-infarct (10 ± 4 % vs. 10 ± 5 %; P = 0.70), total infarct size (24 ± 10 % vs. 21 ± 12 %; P = 0.62) and transmural extent (3.19 ± 2.41 vs. 2.97 ± 2.76; P = 0.70) of the infarct (Fig. 2). According to the regional scar score, left anterior descending scar score (1.55 ± 0.81 vs. 1.08 ± 0.84; P = 0.02) was significantly higher in the primary prevention group (Table 2).
CMR results in subgroup of patients with LVEF ≤ 35 %
In order to correct for the difference in LVEF between the primary and secondary prevention group (Table 2), patients with an LVEF ≤ 35 % were included in a subanalysis. There was no difference in demographics and baseline characteristics of the remaining 60 and 20 patients, respectively. According to LV geometry and function as well as CE-CMR assessed infarct tissue characterization, no significant differences were observed between primary and secondary prevention patients with an LVEF ≤ 35 % (Table 2).
CMR results according to infarct localization
As infarct localization differed between the primary and secondary prevention group (Tables 1 and 2), patients were stratified according to infarct localization. Between the 33 and 10 patients with anterior wall MI, respectively, there was no significant difference in LV dimensions, LV function, or infarct tissue characteristics (Table 3). Among patients with non-anterior infarct localization, the primary prevention group (n = 18) showed a lower LVEF (22 ± 9 % vs. 31 ± 14 %; P = 0.04) and a higher WMSI (2.10 ± 0.52 vs. 1.47 ± 0.50; P < 0.01) than the secondary prevention group (n = 14); but there was no significant difference in infarct tissue characteristics (Table 3).
Follow-up
During 21 ± 9 months of follow-up, 4 patients in the primary prevention group and 4 patients in the secondary prevention group died, respectively. All 8 patients died on heart failure. The frequency of appropriate ICD therapy differed significantly between the primary and secondary prevention group (3/66 (5 %) vs. 9/29 (31 %); P < 0.01). In the primary prevention group, only appropriate shock therapy (2 on VF, 1 on VT) was delivered, while in the secondary prevention group both appropriate shock therapy (n = 3; all on VT) and antitachycardia pacing (n = 6) were delivered. All but 2 patients with appropriate ICD therapy (both secondary prevention) had a ≤35 %.
Patients with appropriate ICD therapy did not differ from patients without event in peri-infarct size (8.83 ± 3.20 % vs. 10.51 ± 4.39 %; P = 0.20), but core infarct size was smaller in patients with events (8.00 ± 4.93 % vs. 12.58 ± 7.37 %; P = 0.040).
The association between infarct tissue characteristics with MACE did not significantly differ among groups (ICD for primary prevention vs. ICD for secondary prevention) (P = 0.25–0.91).
Discussion
The implantation of ICD in MI patients provides protection from SCD following VA. When current guidelines are followed, <1 out of 4 primary prevention ICD recipients experiences actual life-threatening VA requiring shock therapy during a follow-up period of almost 4 years [5]. This shows that there may be some room for improvement in the selection of ICD candidates in the setting of primary prevention.
The infarct core and heterogeneous zone, as well as presence of transmural infarction may serve as an anatomic pathway for reentry, and consequently, the occurrence of VA [10–13, 23, 24]. In this respect, it has recently been demonstrated that a larger size of infarct heterogeneity is related to increased ventricular irritability by programmed electrical stimulation as well as spontaneous VA [8, 9].
While there was a difference in frequency of applied ICD therapy between primary and secondary prevention patients in our study, there was no difference in the size of the infarct tissue characteristics between these two subpopulations of patients. These findings may question the importance of the size of infarct tissue characteristics as a predictor of life-threatening VA [25]. However, size of infarct tissue characteristics is not really all that matters, as it has been demonstrated that a substantial portion of tachycardia originates from reentry occurring in a very small circuit extending just over a few millimeters [26]. Other factors than anatomic substrate may interfere with the risk of VA in the setting of MI; an example may be genetic factors. In this respect, recently, a genome-wide association study identified in patients with a first MI a gene locus prone for ventricular fibrillation [27].
In the primary prevention group, we found a substantially larger amount of MI tissue in the anterior wall of the LV during CMR assessment. Several clinical studies observed that patients with anterior MI usually have a worse LVEF [28]. The larger amount of anterior MI in the primary prevention group may thus actually be expected, as LVEF below 35 % is used as a major risk stratifier for primary prevention with ICD, according to current guidelines. In addition, in our primary prevention group (P < 0.01) there was a higher use of diuretics for symptomatic treatment of heart failure. On the other hand, secondary prevention patients—patients who already had a life-threatening VA in the past—showed more appropriate ICD therapies during follow-up, as may be expected based on the difference in indication. Thus, there must be other factors than the studied CMR characteristics involved to make the myocardium prone to the development of life-threatening VA.
With current clinically applied CE-CMR technology, spatial resolution imposes constraints on what type of tissue is concealed within the peri-infarct zone, characterized by intermediate signal intensities [29]. High-resolution CE-CMR imaging with 1,000-fold higher resolution than clinical scans may bear the potential to obtain further insights in an experimental setting [30]. There is a lack of well-defined gold standard formula for the assessment of infarcted myocardium. Partial volume effects and blurred images by cardiac motion during image acquisition may lead to a relative increase of signal intensity in pixels of the border zone of infarcted compared to remote myocardium, which may lead to an overestimation of the total scar score. Initial visual assessment, manual tracing of endo and epicardial contours, visual identification of the region of interest with maximum signal intensity, and visual check for erroneous inclusion of other regions with high signal intensity (e.g. in/folding or motion artefacts, fat, or pericardial effusion) [31] require experience and involve a certain degree of interpretation. The subjectivity involved can only be minimized by an optimized training of experienced analysts. Finally, signal intensity analysis with current CE-CMR techniques do not incorporate areas of microvascular obstruction, which are hypo-enhanced in CE-CMR [31], which may lead to underestimation of infarct size. As only one patient of the present patient population showed microvascular obstruction, the microvascular incorporated to obstruction area was not the infarct core.
While CMR is the gold standard for the assessment of LV function, myocardial viability, extent and transmurality of scar, our findings suggest that infarct tissue analysis with the CE-CMR technique that is currently applied in clinical practice does not appear to have the potential to improve the current practice of risk stratification in MI patients considered for ICD implantation.
Limitations
Our study comprises a limited number of patients; nevertheless, this represents a consecutive series of patients examined with CE-CMR for that indication. While the secondary outcome of defibrillator shocks was prospectively collected and analyzed, the primary comparison of CE-CMR image characteristics was based on a cross-sectional approach. In the light of the duration of clinical follow-up of 21 ± 9 months, event rates in subgroups should be interpreted carefully. In addition, primary aim of the present study was the assessment of potential differences in infarct tissue characteristics between ICD recipients for primary versus secondary prevention.
Conclusion
There was no difference in infarct tissue characteristics between recipients of ICD for primary versus secondary prevention, while the secondary prevention group showed a higher frequency of applied ICD therapy for ventricular arrhythmia.
Abbreviations
- VA:
-
Ventricular arrhythmia
- SCD:
-
Sudden cardiac death
- MI:
-
Myocardial infarction
- ICD:
-
Implantable cardioverter-defibrillator
- LVEF:
-
Left ventricular ejection fraction
- CMR:
-
Cardiovascular magnetic resonance
- CE:
-
Contrast enhancement
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- EDWM:
-
End-diastolic wall mass
- WMSI:
-
Wall motion score index
- EDWT:
-
End-diastolic wall thickness
- MACE:
-
Major cardiovascular event
References
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M et al (2006) ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114(10):e385–e484
A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med 1997 337(22):1576–1583
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R et al (2005) Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352(3):225–237
Kuck KH, Cappato R, Siebels J, Ruppel R (2000) Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation 102(7):748–754
Strauss DG, Selvester RH, Lima JA, Arheden H, Miller JM, Gerstenblith G et al (2008) ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol 1(5):327–336
Kraaier K, Verhorst PM, van Dessel PF, Wilde AA, Scholten MF (2009) Towards a better risk stratification for sudden cardiac death in patients with structural heart disease. Neth Heart J 17(3):101–106
Kraaier K, van Dessel PF, van der Palen J, Wilde AA, Scholten MF (2009) ECG quantification of myocardial scar does not differ between primary and secondary prevention ICD recipients with ischemic heart disease. Pacing Clin Electrophysiol 33(2):192–197
Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA et al (2009) Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging 2(3):183–190
Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK et al (2007) Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 115(15):2006–2014
Bolick DR, Hackel DB, Reimer KA, Ideker RE (1986) Quantitative analysis of myocardial infarct structure in patients with ventricular tachycardia. Circulation 74(6):1266–1279
Cardinal R, Vermeulen M, Shenasa M, Roberge F, Page P, Helie F et al (1988) Anisotropic conduction and functional dissociation of ischemic tissue during reentrant ventricular tachycardia in canine myocardial infarction. Circulation 77(5):1162–1176
Karagueuzian HS, Fenoglio JJ Jr, Weiss MB, Wit AL (1979) Protracted ventricular tachcardia induced by premature stimulation of the canine heart after coronary artery occlusion and reperfusion. Circ Res 44(6):833–846
Saeed M, Bremerich J, Wendland MF, Wyttenbach R, Weinmann HJ, Higgins CB (1999) Reperfused myocardial infarction as seen with use of necrosis-specific versus standard extracellular MR contrast media in rats. Radiology 213(1):247–257
Pascale P, Schlaepfer J, Oddo M, Schaller MD, Vogt P, Fromer M (2009) Ventricular arrhythmia in coronary artery disease: limits of a risk stratification strategy based on the ejection fraction alone and impact of infarct localization. Europace 11(12):1639–1646
Raitt MH, Klein RC, Wyse DG, Wilkoff BL, Beckman K, Epstein AE et al (2003) Comparison of arrhythmia recurrence in patients presenting with ventricular fibrillation versus ventricular tachycardia in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. Am J Cardiol 91(7):812–816
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS et al (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346(12):877–883
Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW et al (2004) Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 110(25):3760–3765
van Welsenes GH, van Rees JB, Borleffs CJ, Cannegieter SC, Bax JJ, van Erven L et al (2011) Long-term follow-up of primary and secondary prevention implantable cardioverter defibrillator patients. Europace 13(3):389–394
Thygesen K, Alpert JS, White HD, On behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction (2007) Universal definition of myocardial infarction. Eur Heart J 28:2525–2538
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542
Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R et al (2004) Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 44(12):2383–2389
Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ et al (2007) Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol 100(6):930–936
Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F et al (2006) Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol 98(8):1033–1040
Yokota H, Heidary S, Katikireddy CK, Nguyen P, Pauly JM, McConnell MV et al (2008) Quantitative characterization of myocardial infarction by cardiovascular magnetic resonance predicts future cardiovascular events in patients with ischemic cardiomyopathy. J Cardiovasc Magn Reson 10(1):17
Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G et al (2005) Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol 45(7):1104–1108
de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE et al (1988) Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation 77(3):589–606
Bezzina CR, Pazoki R, Bardai A, Marsman RF, de Jong JS, Blom MT et al (2010) Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat Genet 42(8):688–691
Wu Y, Chan CW, Nicholls JM, Liao S, Tse HF, Wu EX (2009) MR study of the effect of infarct size and location on left ventricular functional and microstructural alterations in porcine models. J Magn Reson Imaging 29(2):305–312
Zeppenfeld K, van der Geest RJ (2011) The infarct characteristics on magnetic resonance imaging and ventricular tachycardia: do we see what we need to see? Europace 13(6):770–772
Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P et al (2010) Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging 3(6):743–752
Bondarenko O, Beek AM, Hofman MB, Kuhl HP, Twisk JW, van Dockum WG et al (2005) Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 7(2):481–485
Wong DT, Leung MC, Richardson JD, Puri R, Bertaso AG, Williams K et al (2012) Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: a comparison of angiographic and cardiac magnetic resonance derived measurements. Int J Cardiovasc Imaging. doi: 10.1007/s10554-012-0021-9
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Olimulder, M.A.G.M., Kraaier, K., Galjee, M.A. et al. Infarct tissue characterization in implantable cardioverter-defibrillator recipients for primary versus secondary prevention following myocardial infarction: a study with contrast-enhancement cardiovascular magnetic resonance imaging. Int J Cardiovasc Imaging 29, 169–176 (2013). https://doi.org/10.1007/s10554-012-0077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0077-6