Abstract
In patients with ischemic cardiomyopathy, coronary artery bypass grafting (CABG) offers an important therapeutic option but is still associated with high perioperative mortality. Although previous studies suggest a benefit from revascularization for patients with defined viability by a non-invasive technique, the role of viability assessment to determine suitability for revascularization in patients with ischemic cardiomyopathy has not yet been defined. This study evaluates the hypothesis that the use of PET imaging in the decision-making process for CABG will improve postoperative patient survival. We reviewed 476 patients with ischemic cardiomyopathy (LV ejection fraction ≤0.35) who were considered candidates for CABG between 1994 and 2004 on the basis of clinical presentation and angiographic data. In a Standard Care Group, 298 patients underwent CABG. In a second PET-assisted management group of 178 patients, 152 patients underwent CABG (PET-CABG) and 26 patients were excluded from CABG because of lack of viability (PET-Alternatives). Primary endpoint was postoperative survival. There were two in hospital deaths in the PET-CABG (1.3%) and 30 (10.1%) in the Standard Care Group (P = 0.018). The survival rate after 1, 5 and 9.3 years was 92.0, 73.3 and 54.2% in the PET-CABG and 88.9, 62.2 and 35.5% in the Standard Care Group, respectively (P = 0.005). Cox-regression analysis revealed a significant influence on long-term survival of patient selection by viability assessment via PET (P = 0.008), of LV-function (P = 0.017), and age >70 (P = 0.016). Preoperative assessment of myocardial viability via PET identifies patients, who will benefit most from CABG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with advanced coronary artery disease (CAD) and severely reduced LV-function, coronary artery bypass grafting (CABG) offers an important therapeutic option [1–4] Nevertheless, CABG in this specific group of patients is still associated with a high perioperative mortality that ranges from 4.6 to 20% depending on LV-function and severity of congestive heart failure [2, 4]. On the other hand, LV function can improve significantly after revascularization [5–8]. Clinicians face the difficulty to balance the potential benefit of surgical revascularization with the increased perioperative risk in this specific group of patients [9]. In order to improve mortality, methods are sought to select patients who may benefit mostly from CABG.
The assessment of myocardial viability by nuclear imaging techniques has become an important aspect of the diagnostic and prognostic work-up of patients with ischemic cardiomyopathy [10–14]. Noninvasive imaging, such as positron emission tomography (PET), has been reported to be a useful tool for the determination of tissue viability and hence for the prediction of reversibility of regional LV dysfunction [15]. PET, using nitrogen-13 (N-13) ammonia and Fluorine-18 fluorodeoxyglucose (F-18 FDG) is a well established method to further differentiate viable tissue that may benefit from revascularization from scarred myocardium [12–13].
Previous studies showed that revascularization of patients with viability results in an improvement of heart failure symptoms, and exercise capacity [16–17]. Patients selected for CABG on the basis of PET viability studies may also have fewer perioperative complications [18]. A meta-analysis from 2002 including 3,088 patients suggests that the differentiation of viable from nonviable myocardium is also an important issue in the selection process between medical therapy versus myocardial revascularization in heart failure patients [10]. Nevertheless, the role of viability assessment to determine suitability for revascularization is still an open question and an optimal diagnostic protocol in patients with ischemic cardiomyopathy has not yet been defined. In a recent study, Beanlands et al. [19] could not demonstrate a significant reduction in cardiac events in patients with LV-dysfunction and suspected coronary disease for FDG PET-assisted management versus standard care.
The current study evaluates the hypothesis that the use of PET imaging in the decision-making process for CABG will improve postoperative patient survival. In a retrospective study, 476 consecutive patients with ischemic cardiomyopathy were analyzed who were referred for CABG between 1994 and 2004. Postoperative survival was compared in patients selected for revascularization on the basis of clinical and angiographic data alone, and patients who underwent a supplementary myocardial viability testing via PET.
Materials and methods
The current study had the approval of the local Ethic Committee of the Technische Universitaet Muenchen, Munich, Germany, (2370/09). We reviewed 501 consecutive patients with ischemic cardiomyopathy (LV ejection fraction ≤0.35) who were considered candidates for CABG between 1994 and 2004. Firstly, 25 patients, who were referred from overseas, were excluded due to the impossibility of a sufficient follow-up, and 476 patients were finally included in the current study. A standardized questionnaire was sent to all patients. If no answer ensured, follow-up was obtained by telephone interview and/or further information was requested from registry offices. Thus, follow-up could be completed in 100% of the patients. Perioperative complications and mortality were recorded prospectively as part of an ongoing quality assurance program. In-hospital mortality was defined as death within 30 days after operation.
Study groups
Cardiac catheterization was performed in all patients to assess ventricular function and extent of coronary artery disease (CAD). Global LV-function was measured by biplane cine-angiography. The patients who were candidates for CABG were divided into two groups (Fig. 1): A Standard Care Group of 298 patients who did not have viability testing preoperatively. A second group of 178 patients underwent PET assisted management: 152 patients had sufficient viability according to PET and underwent CABG (PET-CABG), whereas 26 patients had no sufficient viability and were selected to medical treatment (n = 18) or transplantation (n = 8).
476 candidates for CABG 1994-2004. Patients were selected for CABG on the basis of clinical presentation and angiographic data (n = 298, Standard Care Group) or on the basis of an additional assessment of the extent of viable tissue by PET (n = 178). 152 patients of the latter group underwent CABG (PET-CABG) and 26 patients were excluded from CABG because of lack of viability (PET-Alternatives) and either underwent heart transplantation (n = 8) or received medical treatment only (n = 18)
PET studies
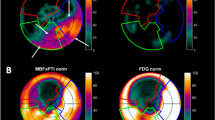
All patients without known diabetes mellitus were studied in the postprandial state after additional oral glucose loading with 50 g of glucose. Patients with known diabetes or abnormal glucose tolerance received insulin before and during the imaging sequence, according to a standardized protocol [20]. After initial transmission scanning for attenuation correction, rest regional myocardial perfusion imaging with N-13 ammonia (740 MBq) was performed. After sufficient time for N-13 decay, F-18 FDG (370 MBq) was injected, and data acquisition was initiated 40 min after tracer injection. Transaxial planes were obtained using whole-body PET (Siemens CTI 951 or Siemens Exact 47). Attenuation-corrected transaxial PET images were generated from N-13 ammonia and F-18 FDG data. The images were reoriented perpendicular to the long axis of the left ventricle, after which volume-weighted polar maps were calculated from circumferential profiles of the maximal myocardial activity. In addition, the transaxial image data were realigned to generate images in short-axis, vertical and horizontal long-axis views for visual analysis [21]. Regional tracer uptake of N-13 ammonia and F-18 FDG was evaluated visually, by two experienced observers who had no knowledge of the clinical and angiographic data, to estimate the extent of necrosis and viable tissue (Fig. 2). The time between viability testing and CABG was in all cases less than 3 months.
PET-Studies and Viability Assessment. Attenuation-corrected transaxial PET images were generated from N-13 ammonia and F-18 FDG data. The images were reoriented perpendicular to the long axis of the left ventricle, after which volume-weighted polar maps were calculated from circumferential profiles of the maximal myocardial activity
Viability criteria
Tissue viability by PET was assessed by the combined interpretation of perfusion and metabolism within the vascular territories of the left ventricle. The septal, anterior and anterolateral walls were considered the vascular territory of the left anterior descending coronary artery. The left circumflex coronary artery was considered to supply the lateral and posterolateral walls, whereas the vascular territory of the right coronary artery was the inferior and posterior walls. Two different viability criteria were used: (1) reduced blood flow with preserved or increased F-18 FDG uptake (mismatch); (2) normal or near-normal blood flow with normal or increased F-18 FDG uptake (normal). Reduced blood flow with reduced F-18 FDG uptake (matched defect) was used as the criterion for scar (Fig. 2). On the basis of this visual evaluation, three main criteria were used to determine whether an individual patient was a suitable candidate for CABG: (1) The presence of a “normal or mismatch” pattern in akinetic or severely hypokinetic myocardial areas supplied by a stenosed or obstructed artery was required. (2) If viable myocardium was detected in at least two different vascular territories, we considered the patient an adequate candidate for CABG, independent of the estimated target vessel size from the angiographic report. (3) A large area of scar tissue using an approximate threshold of 40% was a deciding factor against CABG. This arbitrary threshold was based on studies of acute myocardial infarction, that indicated a high incidence of cardiogenic shock in infarct areas >40% of LV mass [22–24]. It was assumed that patients with a large infarct area are more susceptible to hemodynamic complications during CABG. The visually estimated extent of scar tissue was retrospectively confirmed by semiquantitative analysis. Scar tissue was defined as F-18 FDG uptake ≤50% of maximal uptake on “bull’s eye” quantitation [25]. On the basis of the PET criteria, in association with the angiographic report, 26 patients were found to be inappropriate candidates for CABG and either underwent heart transplantation (n = 8) or received only medical treatment (n = 18).
Statistical analysis
The student t-test for two independent samples and the chi-squared test were used for continuous and categorical outcomes, respectively, to evaluate differences between the PET-CABG and the Standard Care Group. A two-sided P value <0.05 was considered statistically significant. Kaplan–Meier survival curves were calculated to estimate long-term survival; differences between groups were assessed with the log-rank test. Multiple Cox regression analysis was performed to assess the impact of the following possible risk factors simultaneously: viability, LV-function, diabetes, sex and age.
Results
Pre- and intraoperative patient data
Table 1 depicts the main patient characteristics of both groups. No differences were seen with regard to preoperative NYHA status, reoperation, diabetes, the presence of sinus rhythm, preoperative angina, preoperative creatinine, COPD, or prior myocardial infarction. Differences were observed regarding LV-function, age and gender. The PET-CABG exhibited a lower LV-function (PET-CABG: 26.0 ± 6.1, Standard Care Group: 28.1 ± 5.3; P < 0.001; Fig. 2), a lower percentage of patients >70 years in the PET-CABG (PET-CABG: 30.3%, Standard Care Group: 39.9%; P = 0.044; Table 1) and the lower percentage of woman in the PET-CABG (PET-CABG: 10.5%, Standard Care Group: 17.8%; P = 0.043) as opposed to the Standard Care Group. 450 patients finally underwent CABG, whereas 26 patients were not selected for revascularization due to insufficient viability as defined previously. 18 patients of these group received medical treatment and 8 patients underwent heart transplantation (Fig. 1). No differences were seen between both groups regarding cardiopulmonary bypass time (PET-CABG: 103.9 min ± 33.6; Standard Care Group: 98.8 min ± 31.7; P = 0.128) and number of coronary anastomoses per patient (PET-CABG: 3.3 ± 1.0; Standard Care Group: 3.2 ± 0.9; P = 0.264) .
Follow-up
Mean follow-up was 3.8 years ± 3.02, range from 2 days to 11.07 years (group A: 4.9 ± 2.9, range from 1 day to 10.1 years; group B: 3.3 ± 2.9, range 0.002–11.1 years; PET-Alternatives: 3.16 ± 3.1, range from 0.02 to 10.9 years) (Fig. 1). Survival analysis was calculated according to Kaplan–Meier and a log-rank test was performed (Fig. 1). The log-rank test between the PET and the Standard Care Group showed also significant difference (p = 0.0052). There were two in hospital deaths in the PET-CABG (1.3%) and 30 (10.1%) in the Standard Care Group (P = 0.018). The survival rate after 1, 5 and 10 years were in the PET-CABG 92.0, 73.3 and 54.2% and in the Standard Care Group 88.9, 62.2 and 35.5%, respectively (P = 0.005). In the group of PET-Alternatives, survival rate was 61.5% (0.8 years), and 29.2% (4.8 years). Cox-regression analysis revealed an influence of preoperative viability assessment via PET (P = 0.008), of preoperative LV-function (P = 0.017), and age >70 (P = 0.016) on long-term survival. Diabetes (P = 0.072) and female gender (P = 0.085) had no significant influence (Table 2; Fig. 3).
Discussion
Although surgical revascularization remains an important therapeutic option in ischemic cardiomyopathy, these patients face high perioperative mortality when undergoing CABG that ranges from 4.6 to 20% depending on LV-function, comorbidities and severity of congestive heart failure [2, 4, 26]. Previous studies found a benefit from revascularization for patients with defined viability by a non-invasive technique, so that preoperative patient selection by viability testing has become an issue [10, 11, 21, 27–29].
To assess myocardial viability, different diagnostic methods are currently performed, i.e. FDG/PET, MRI, SPECT and echocardiography whereas FDG/PET is considered the gold-standard due to its ability to differentiate dysfunctional but viable myocardium (hibernating myocardium) from scar formation and normal myocardium [14, 30]. In a multi-centre study including 157 patients, Gerber et al. [31] showed a high sensitivity and moderate specificity of FDG/PET to predict improvement of cardiac function after coronary revascularization. The improvement of LV-function after revascularization seems to be directly related to the number of dysfunctional but viable segments, i. e. the mass of viable tissue [32–35]. Furthermore, Tarakji et al. [36] described a strong association between early revascularization and survival in a large series of 765 patients who underwent comprehensive PET imaging.
A meta-analysis from 2002 included 3,088 patients published in studies examining survival with revascularization versus medical therapy after myocardial viability testing in patients with ischemic cardiomyopathy [10]. Non-invasive imaging techniques included thallium perfusion imaging, FDG/PET, and dobutamine echocardiography. Viability was interpreted as “present” or “absent” based on individual study definitions. The authors found a strong association between myocardial viability on noninvasive testing and improved survival after revascularization in patients with ischemic cardiomyopathy. Furthermore, it was suggested that the differentiation of viable from nonviable myocardium could be crucial in the selection process between medical therapy versus myocardial revascularization.
Nevertheless, the role of viability assessment to determine suitability for revascularization is still an open question and an optimal diagnostic protocol in patients with ischemic cardiomyopathy has not yet been defined. Recently, in the PARR-2 study (Positron emission tomography and recovery following revascularization), Beanlands et al. included patients with ischemic cardiomyopathy and randomized the patients to management assisted by FDG PET (n = 218) or standard care (n = 212). The study found a reduction of adverse cardiac events of 36% in the standard care arm versus 30% in the PET-assisted arm that did not reach statistical significance [19]. This study has been criticized for the fact, that 25% patients with PET-indicated revascularization did not have it done [37]. In the subgroup of patients who adhered to PET recommendations regarding revascularization, however, significant survival benefits were observed. These findings are supported by the current study, in which every patient with sufficient viability in the PET-assisted group underwent CABG and exhibited significant better mortality rates after revascularization.
The concept of a preoperative PET-based selection of patients who benefit mostly from CABG was examined by Haas et al. [18] who found a significant reduction in perioperative mortality in patient with defined viability. Subsequent studies indicate that dysfunctional regions with normal perfusion are more common than mismatch, have less associated tissue injury and are more likely to demonstrate complete recovers than mismatch segments (31 vs. 18%, respectively) [38–39].
The key finding of the present study was the significant reduction of the 30-day mortality in the PET-CABG group with 1.3 versus 10.3% in the Standard Care group. Despite the improvement of hospital mortality after CABG in the last years, the observed early mortality rate of 1.3% in the PET-CABG group is lower than current reports by the STICH (Surgical Treatment for Ischemic Heart Failure) study [3] or the study by Nardi et al. [26], that reported a hospital mortality of 5 and 5.3%, respectively. The early survival benefit of the PET-CABG persists in the long-term as reflected by the superior survival of the PET-CABG over a 10 year follow-up.
A preoperative selection protocol, based on myocardial viability testing via PET identifies patients who can undergo CABG with a risk profile that is comparable to CABG in patients with normal LV-function. The Standard Care Group did not undergo a selection process via PET, and presumably patients with greater unrecognized extent of scar tissue were not excluded.
The selection process in the present study leads to a proper identification of patients with a lower risk profile when undergoing CABG. Statistical analysis revealed the selection process itself as a significant prognostic factor for postoperative survival (Table 2). FDG/PET offers unique information beside clinical and angiographic date that leads to improved patient selection, which subsequently results in improved postoperative recovery with a low early mortality and superior long-term survival after CABG. An important limitation of this study is its retrospective design. The preoperative scheduling for viability testing was based on an intention-to-treat basis, but not in a prospective, randomized manner, so that a potential bias cannot be totally excluded.
Previous studies have addressed the issue of patient selection for revascularization in ischemic cardiomyopathy due to the high perioperative risk. Nevertheless, former studies lack long-term follow-up as well as sufficient sample size to analyze potential benefits of viability testing prior revascularization. Additional arguments have been discussed: According to subgroup analysis of the PARR-2 study, the adherence to PET recommendations remains crucial [19]. In the PET-assisted group of the current study, PET recommendations were consequently followed in all cases.
Timing of revascularization has become another issue of recent research [36], suggesting a benefit from early revascularization. In the current study, patients were already candidates for CABG when viability testing was performed and subsequently every patient of the PET-assisted group underwent surgery in less than 3 month time after PET. Furthermore, the results of the current study are in line with the PARR-1study that found the amount of scar detected by FDG-PET as a significant independent predictor of LV function recovery after revascularization [40].
The main limitation of the current study is its retrospective design. The study included patients with severe reduction of LV function who were referred for revascularization. Limited availability of PET and outside referral prohibited prospective randomization of patients in this study. Although a randomization protocol would have been ideal, careful retrospective analysis of risk factors was performed in both groups to identify any selection bias. The decision for an additional viability testing was made by the responsible surgeon. Both groups suffer equally from angina and are in NYHA III + IV (Table 1). Important risk factors for perioperative mortality, such as preoperative renal function, diabetes, prior myocardial infarction, chronic obstructive pulmonary disease, prior cardiac surgery or diabetes are comparable in both groups (Table 1). Furthermore, intraoperative parameters like cardiopulmonary bypass time or the number of anastomoses per patient did not differ between the two groups. Only small differences between the two groups were seen in LVEF, age, and gender (Table 1).
The present study did not compare FDG-PET with other imaging modalities for detection of viable myocardium. Further studies are necessary to determine whether the same results may be obtained with conventional scintigraphic techniques, such as thallium-201 imaging, magnetic resonance imaging or low dose dobutamine echocardiography.
Nevertheless, the cut-off point of 40% of scar tissue for the indication for CABG, which was applied in the present study, represents an arbitrary threshold. This arbitrary threshold was based on studies of acute myocardial infarction that indicated a higher incidence of cardiogenic shock in infarct areas averaging 37, 43 and 51% of LV mass [22–24] which may indicate an irreversible condition. Yoshida et al. [24] showed that the size of the infarct area and viability in arterial zones at risk assessed by PET are good prognostic markers for mortality. In their study, 6 of 35 patients had an infarct size between 39 and 77%, as determined by quantitative PET measurements. Four of these patients died within a 3-year period, three of them after revascularization.
As the present study showed, the criterion of scar extent alone is not sufficient for the selection process in some patients. Four patients in the PET-CABG exhibited a scar tissue area ≥40%. However, in these patients the other main viability criteria and the angiographic report supported the decision, that these patients were adequate candidates for CABG. Two of the patients are still alive (follow-up time: 6.7 and 7.7 years). In both patients, PET revealed high percentages of viable myocardium: 40–45% scar, 52–60% viable myocardium and 0–3% mismatch. A third patient died 8 months after surgery (PET: 46% scar, 5% mismatch and 49% viable myocardium), and the fourth patient died 3.9 years after CABG (PET: 43% scar, 10% mismatch, 47% viable myocardium). These findings underscore the complexity of decision making in this specific group of patients. Nevertheless, only four patients of 178 patients (2.24%), who underwent preoperative PET management, did not totally apply to the exclusion criterion by PET. Therefore, we suggest that in patients with a scar tissue area of around 40%, the 40% cut-off point should not be strictly applied but appreciated in request to the other viability criteria as well as angiographic results.
Conclusions
In ischemic cardiomyopathy, patient selection by preoperative viability testing via PET leads to a significant reduction of perioperative mortality rates after surgical revascularization. This survival benefit persists in the long-term. Prospective, randomized are necessary to further evaluate the impact of preoperative viability assessment in this high risk group of patients.
References
ACC/AHA (2004) Guideline update for coronary artery bypass graft surgery. Circulation 110(14):e340–e437
Appoo J, Norris C, Merali S et al (2004) Long-term outcome of isolated coronary artery bypass surgery in patients with severe left ventricular dysfunction. Circulation 110(11 suppl 1):11–13
Jones RH, Velazquez EJ, Michler RE et al (2009) Coronary Bypass Surgery with or without Surgical Ventricular Reconstruction. N Engl J Med 360(17):1705–1717
Westaby S (2004) Coronary revascularization in ischemic cardiomyopathy. Surg Clin North Am 84(1):179–199
Bax JJ, Maddahi J, Poldermans D et al (2003) Preoperative comparison of different noninvasive strategies for predicting improvement in left ventricular function after coronary artery bypass grafting. Am J Cardiol 92(1):1–4
Bax JJ, Visser FC, Poldermans D et al (2001) Relationship between preoperative viability and postoperative improvement in LVEF and heart failure symptoms. J Nucl Med 42(1):79–86
Bax JJ, Visser FC, Poldermans D et al (2001) Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation 104(12 Suppl 1):I314–I318
Kang WJ, Lee DS, Paeng JC, Kim KB, Chung JK, Lee MC (2003) Prognostic value of rest (201) Tl-dipyridamole stress (99 m) Tc-sestamibi gated SPECT for predicting patient-based clinical outcomes after bypass surgery in patients with ischemic left ventricular dysfunction. J Nucl Med 44(11):1735–1740
Gibbons RJ, Chareonthaitawee P, Bailey KR (2006) Revascularization in systolic heart failure: a difficult decision. Circulation 113(2):180–182
Allman KC, Shaw LJ, Hachamovitch R, Udelson JE (2002) Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 39(7):1151–1158
Bax JJ, van der Wall EE, Harbinson M (2004) Radionuclide techniques for the assessment of myocardial viability and hibernation. Heart 90(Suppl 5):v26–v33
Beller GA (2000) Noninvasive assessment of myocardial viability. N Engl J Med 343(20):1488–1490
Sawada SG (2006) Positron emission tomography for assessment of viability. Curr Opin Cardiol 21(5):464–468
Wijns W, Vatner SF, Camici PG (1998) Hibernating myocardium. N Engl J Med 339(3):173–181
Le Guludec D, Lautamaki R, Knuuti J, Bax JJ, Bengel FM (2008) Present and future of clinical cardiovascular PET imaging in Europe–a position statement by the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging 35(9):1709–1724
Di Carli MF, Asgarzadie F, Schelbert HR et al (1995) Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation 92(12):3436–3444
Marwick TH, Zuchowski C, Lauer MS, Secknus MA, Williams J, Lytle BW (1999) Functional status and quality of life in patients with heart failure undergoing coronary bypass surgery after assessment of myocardial viability. J Am Coll Cardiol 33(3):750–758
Haas F, Haehnel CJ, Picker W et al (1997) Preoperative positron emission tomographic viability assessment and perioperative and postoperative risk in patients with advanced ischemic heart disease. J Am Coll Cardiol 30(7):1693–1700
Beanlands RS, Nichol G, Huszti E et al (2007) F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol 50(20):2002–2012
vom Dahl J, Herman WH, Hicks RJ et al (1993) Myocardial glucose uptake in patients with insulin-dependent diabetes mellitus assessed quantitatively by dynamic positron emission tomography. Circulation 88(2):395–404
Eitzman D, al-Aouar Z, Kanter HL (1992) Clinical outcome of patients with advanced coronary artery disease after viability studies with positron emission tomography. J Am Coll Cardiol 20(3):559–565
Alonso, Scheidt S, Post M, Killip T (1973) Pathophysiology of cardiogenic shock. Quantification of myocardial necrosis, clinical, pathologic and electrocardiographic correlations. Circulation 48(3):588–596
Harnarayan C, Bennett MA, Pentecost BL, Brewer DB (1970) Quantitative study of infarcted myocardium in cardiogenic shock. Br Heart J 32(6):728–732
Yoshida K, Gould KL (1993) Quantitative relation of myocardial infarct size and myocardial viability by positron emission tomography to left ventricular ejection fraction and 3-year mortality with and without revascularization. J Am Coll Cardiol 22(4):984–997
Baer FM, Voth E, Deutsch HJ et al (1996) Predictive value of low dose dobutamine transesophageal echocardiography and fluorine-18 fluorodeoxyglucose positron emission tomography for recovery of regional left ventricular function after successful revascularization. J Am Coll Cardiol 28(1):60–69
Nardi P, Pellegrino A, Scafuri A et al (2009) Long-term outcome of coronary artery bypass grafting in patients with left ventricular dysfunction. Ann Thorac Surg 87(5):1401–1407
Desideri A, Cortigiani L, Christen AI et al (2005) The extent of perfusion-F18-fluorodeoxyglucose positron emission tomography mismatch determines mortality in medically treated patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol 46(7):1264–1269
Di Carli MF, Davidson M, Little R et al (1994) Value of metabolic imaging with positron emission tomography for evaluating prognosis in patients with coronary artery disease and left ventricular dysfunction. Am J Cardiol 73(8):527–533
Sawada S, Hamoui O, Barclay J et al (2005) Usefulness of positron emission tomography in predicting long-term outcome in patients with diabetes mellitus and ischemic left ventricular dysfunction. Am J Cardiol 96(1):2–8
Camici PG, Prasad SK, Rimoldi OE (2008) Stunning, hibernation, and assessment of myocardial viability. Circulation 117(1):103–114
Gerber BL, Ordoubadi FF, Wijns W (2001) Positron emission tomography using (18) F-fluoro-deoxyglucose and euglycaemic hyperinsulinaemic glucose clamp: optimal criteria for the prediction of recovery of post-ischaemic left ventricular dysfunction. Results from the European community concerted action multicenter study on use of (18) F-fluoro-deoxyglucose positron emission tomography for the detection of myocardial viability. Eur Heart J 22(18):1691–1701
Bax JJ, Cornel JH, Visser FC (1996) Prediction of recovery of myocardial dysfunction after revascularization. Comparison of fluorine-18 fluorodeoxyglucose/thallium-201 SPECT, thallium-201 stress-reinjection SPECT and dobutamine echocardiography. J Am Coll Cardiol 28(3):558–564
Bax JJ, Maddahi J, Poldermans D et al (2002) Sequential (201) Tl imaging and dobutamine echocardiography to enhance accuracy of predicting improved left ventricular ejection fraction after revascularization. J Nucl Med 43(6):795–802
Slart RH, Bax JJ, van Veldhuisen DJ et al (2006) Prediction of functional recovery after revascularization in patients with coronary artery disease and left ventricular dysfunction by gated FDG-PET. J Nucl Cardiol 13(2):210–219
Tillisch J, Brunken R, Marshall R et al (1986) Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med 314(14):884–888
Tarakji KG, Brunken R, McCarthy PM et al (2006) Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation 113(2):230–237
Gould KL (2007) Not All Randomized Trials Are Equal. J Am Coll Cardiol 50(20):2013–2015
Haas F, Augustin N, Holper K et al (2000) Time course and extent of improvement of dysfunctioning myocardium in patients with coronary artery disease and severely depressed left ventricular function after revascularization: correlation with positron emission tomographic findings. J Am Coll Cardiol 36(6):1927–1934
Haas F, Jennen L, Heinzmann U et al (2001) Ischemically compromised myocardium displays different time-courses of functional recovery: correlation with morphological alterations? Eur J Cardiothorac Surg 20(2):290–298
Beanlands RS, Ruddy TD, deKemp RA et al (2002) Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol 40(10):1735–1743
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Boehm, J., Haas, F., Bauernschmitt, R. et al. Impact of preoperative positron emission tomography in patients with severely impaired LV-function undergoing surgical revascularization. Int J Cardiovasc Imaging 26, 423–432 (2010). https://doi.org/10.1007/s10554-010-9585-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-010-9585-4