Abstract
The purpose of this study was to assess the performance of 0.5 versus 3.0 mm slice reconstructions in depicting coronary calcium with special attention to patients having zero calcium scores at 3.0 mm reconstructions by using computed tomography (CT). Imaging was performed by volumetric 320-detector row CT. Scans of 100 patients with a negative and 100 patients with a positive Agatston score at 3.0 mm reconstructions were consecutively selected. Non-overlapping volume sets with 3.0 and 0.5 mm slice thickness were reconstructed from the same raw data and Agatston and volume scores were obtained. The Wilcoxon signed ranks test was used to determine statistical differences between 3.0 and 0.5 mm calcium scores. Agatston and volume scores obtained at 0.5 mm were significantly higher than at 3.0 mm reconstructions (mean Agatston score: 266 ± 495 vs. 231 ± 461. Mean volume score: 223 ± 399 vs. 206 ± 385, both P < 0.01). In 21% of patients with zero 3.0 mm Agatston scores, a positive Agatston and/or volume score was found at 0.5 mm reconstructions. With volumetric 320-detector row CT, prospective ECG-triggered calcium scoring at 0.5 mm compared to 3.0 mm reconstructions leads to an increase in Agatston and volume scores and small amounts of coronary calcium are earlier depicted. This may be of special interest in patients with zero calcium scores with traditional 3.0 mm measures, where 0.5 mm reconstructions may help in superior depicting or ruling out coronary artery disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery calcification is a direct sign of atherosclerotic coronary artery disease [1, 2] and has shown a strong predictor for the risk of cardiovascular disease or events, including myocardial infarction and/or cardiac death [3–5]. The amount of coronary calcium can be quantified non-invasively by using computed tomography (CT) techniques and calculating the Agatston score [6] or scores such as the volume score [7] or calcium mass [8]. For calculating the Agatston score, a weighing factor is applied based on the peak attenuation within a calcified lesion. This may lead to a higher variability than with the volume score or calcium mass [9]. However, large clinical risk stratification studies are based on the Agatston score [3, 4, 10]. Therefore, in clinical practice, the Agatston score is generally advised and used to identify and stratify patients at risk for coronary artery disease [11–13].
Electron-beam CT (EBCT) has been regarded the standard of reference method for detection and quantification of coronary calcium, and studies indicating the risk for coronary artery disease are based on EBCT investigations [10, 14, 15]. Calcium score investigations are increasingly performed by using multi-detector CT (MDCT) techniques. It has been shown that MDCT calcium scores correlate well with that of EBCT [5, 16–20], although some studies suggest that MDCT tends to underestimate calcium scores as compared to EBCT [20, 21].
Disadvantage of 3.0 mm slice reconstructions, traditionally used with EBCT, is the presence of partial volume errors. This may lead to inaccuracies in evaluating the actual presence of coronary calcium and may cause an underestimation of small or low attenuation calcifications [22] and a high interscan variability [18, 23].
Guidelines and recommendations for use of calcium scores in clinical practice have recently been published by the European Society of Cardiac Radiology (ESCR) and the North American Society for Cardiovascular Imaging (NASCI) [13]. In that paper, the authors stress the value of zero calcium scores that excludes most clinically relevant coronary artery disease [13]. With these recommendations and with the increase in the overall number of cardiac MDCT investigations, it is likely that the number of calcium score investigations will further increase.
Recently, a 320-detector-row volumetric MDCT scanner has become available for clinical imaging that allows full cardiac imaging by using a single prospective ECG-triggered 0.35 s rotation [24, 25]. The volume is acquired with 0.5 mm collimation thickness. We hypothesized that coronary calcium would be depicted to better advantage by using thin 0.5 mm slice reconstructions than with standard 3.0 mm slice reconstructions (due to less partial volume effect), and that this might result in the detection of coronary calcium that goes undetected with 3.0 mm slice collimation. Accordingly, the purpose of this study was to assess the performance of 0.5 versus 3.0 mm slice reconstructions in depicting coronary calcium, with special attention to patients having zero calcium scores at 3.0 mm reconstructions.
Materials and methods
Study population
The detection of coronary calcium at 3.0 and 0.5 mm reconstructions was retrospectively evaluated in 200 patients. Hundred patients with a negative Agatston score (=0) and 100 patients with a positive Agatston score (≥1) were consecutively selected from a database of patients who had undergone non contrast-enhanced calcium score CT for clinical indications between February 2008 and April 2009 (127 men, 73 women; mean age 57 ± 11 years; length 177 ± 10 cm; weight 83 ± 13 kg). Patients with coronary stents (n = 12), pacemakers (n = 10), and prosthetic heart valves (n = 24) had been excluded beforehand to avoid scoring artifacts. Another five patients were excluded based on limited diagnostic image quality with 3.0 mm slice reconstructions. In these patients, due to obesity, image quality was limited and calcifications could not be reliably distinguished from image noise. Our institutional review board does not require its approval for anonymous retrospective technical analysis of data, as was the case in this study.
CT protocol
All examinations were performed with a 320-detector row CT scanner (Toshiba Aquilion ONE, Toshiba Medical Systems, Otawara, Japan). To lower the heart rate, 25–100 mg oral Metoprolol was administered in patients with a cardiac frequency exceeding 60 beats per minute and when no contra-indications were present. Mean heart rate during scanning was 56 ± 8 beats per minute. The scan range was planned between the carina and cardiac apex. Depending on the expected scan range, a 320 × 0.5 mm or a 280 × 0.5 mm detector configuration was used. Immediately before image acquisition, an optimal reconstruction phase was automatically determined during a breath hold exercise with ECG-recording by use of cardiac scanning software (SureCardio, Toshiba Medical Systems, Otawara, Japan). Full cardiac calcium score acquisition was performed in a single gantry rotation during breath hold at inspiration that allows image reconstruction at a single cardiac phase. Scan parameters were: tube voltage 120 kV, tube current 200–400 mA (mean 266 ± 33 mA), dependent on patient size and shape as visually assessed by the Radiology Technician: 200 mA for small/thin patients, 250 mA for normal patients, and 300–400 mA for large/obese patients. Rotation time was 0.35 s. Scan parameters were not adapted to compensate for increased image noise with thin slice reconstructions. Effective radiation dose estimation was based on the dose-length product provided by the scanner for each patient and by using the correction factor 0.017 for chest imaging in adults [26]. Estimated dose was 2.0 ± 0.4 mSv.
Image reconstruction
Image reconstruction was performed using a standard reconstruction kernel filter (FC12). Images were reconstructed with a 200–220 mm2 field-of-view. Non-overlapping 3.0 mm datasets were reconstructed, which is the standard method used in clinical practice based on EBCT [6]. Furthermore, an additional non-overlapping 0.5 mm dataset was reconstructed from the same raw data for evaluation of coronary calcium. The reconstructions were transferred to a post-processing workstation for analysis.
Calcium scoring
Evaluating calcium score was performed on the post-processing workstation (Vitrea FX, version 1.0, Vital Images, Minnetonka, USA), using calcium score analysis software (VScore, Vital Images). Coronary calcium was defined as an area of at least three ‘face-connected’ voxels in the axial plane in the course of a coronary artery, with an attenuation threshold-value of ≥130 HU. Three in axial plane face-connected voxels correspond to a minimum lesion area >1 mm2 that is used as reference value in calcium scores [6].
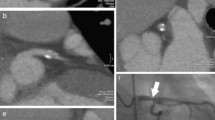
Calcifications were identified in the 3.0 mm dataset first in which all regions with CT attenuation higher than the threshold-value of 130 HU were marked in the course of the coronary arteries. Secondly, calcifications were identified and marked in the 0.5 mm dataset, including visually clearly recognizable calcium on 3.0 mm slices that fell below the threshold value for automatic detection on the 3.0 mm slices. To prevent the false-positive depiction of image noise as calcifications, direct lesion comparison was used by scoring the 0.5 and 3.0 mm datasets side by side. If suspected coronary calcium was identified in the 0.5 mm dataset, this was marked if both the following criteria were met: the size of the identified lesion had to be larger than spots within the same slice outside the coronary arteries that also reached the attenuation threshold, and the lesion had to be present in at least two adjacent slices (Fig. 1).
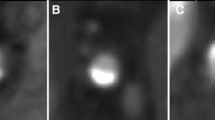
Calcium score in a 57-year old male with a zero calcium score at 3.0 mm slice reconstructions (not shown). Calcium in the left anterior descending artery exceeding the threshold value of 130 HU (arrowheads) is shown in two adjacent slices (a, b) and is larger in size than image noise shown in the ascending aorta on the same slice level (white arrows). A more detailed view is shown in c, d. The Agatston score of the lesion with 0.5 mm reconstructions was 13
The Agatston score [6] and volume score [7] were obtained in all scans. The calcium scores obtained with the 0.5 mm dataset were automatically corrected for slice thickness by a factor of 0.5/3.0. The patients were categorized in different risk groups according to the absolute amount of calcium based on the Agatston score [11]. Although this risk stratification scheme does not account for patient age, gender and race, it has been suggested that absolute calcium scores predict cardiovascular events better than adjusted percentiles [27, 28]. Analysis was performed by one investigator with 2 years of experience in cardiac CT and supervised by a radiologist with 7 years experience in cardiac CT.
Statistical analysis
Statistical analysis was performed using SPSS for Windows, version 16.0 (Chicago, Illinois). The mean, standard deviations and median values were calculated for the calcium scores obtained with the 3.0 and 0.5 mm datasets, and the absolute differences were calculated. The Wilcoxon signed ranks test was applied to determine statistical significant differences between the 3.0 and 0.5 mm scores and between the change in Agatston and volume scores. A P-value of less than 0.05 was considered statistically significant.
Results
Negative calcium score (0)
In 21 of 100 patients with a negative Agatston score at 3.0 mm reconstructions (21%), calcium spots were identified at 0.5 mm reconstructions. Of these 21 patients, 18 had a positive Agatston and a positive volume score with 0.5 mm reconstructions (mean Agatston score ± SD: 3 ± 3. Mean volume score ± SD: 5 ± 4; Fig. 2). Additionally, three patients had only a volume score of 1 at the 0.5 mm reconstructions. The distribution of 0.5 mm Agatston and volume scores of patients with a negative Agatston score at 3.0 mm reconstructions is shown in Table 1.
Calcium score in a 59-year old male with 3.0 mm (a) and 0.5 mm slice reconstructions. The Agatston score obtained at 3.0 mm reconstructions was zero as the visible calcium spot fell below the threshold value. With 0.5 mm slice reconstruction, the calcified lesion identified in the left anterior descending artery resulted in an Agatston score of 5
Positive calcium score (≥1)
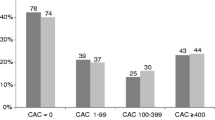
Of the patients with a positive calcium score, Agatston scores obtained at 0.5 mm (mean ± SD; 266 ± 495) were statistically significant higher than at 3.0 mm reconstructions (mean ± SD; 231 ± 461, P < 0.01; Fig. 3). The distribution of patients within different Agatston risk groups according to the classification of Rumberger et al. [11] is shown in Table 2. As expected, the absolute differences in Agatston score between the 3.0 and 0.5 mm reconstructions stratified per Agatston risk group increases with the increase in risk category (Table 2). Furthermore, volume scores obtained at 0.5 mm were statistically significant higher than the scores obtained at 3.0 mm reconstructions (mean ± SD: 223 ± 399 vs. 206 ± 385, P < 0.01). Overall, the change in the volume scores for 0.5 versus 3.0 mm reconstructions was less pronounced than the change in Agatston scores.
Calcium score of a 47-year old male with 3.0 mm (a) and 0.5 mm slice reconstructions (b). Identification of a calcified lesion in the left anterior descending artery at 0.5 mm reconstruction with an Agatston score of 9 (b, arrow), that fell below the threshold value for detection at the 3.0 mm reconstruction (a, arrow)
Total group
In 112 of 200 patients a higher Agatston score was found at 0.5 mm than at 3.0 mm slice reconstructions (mean ± SD: 222 ± 475 vs. 190 ± 440, mean difference 33, P < 0.01). Seven patients had higher Agatston scores at 3.0 mm than with 0.5 mm reconstructions (mean ± SD: 177 ± 203 vs. 188 ± 151, mean difference 11, P = 0.02). The change in Agatston score would have led to a shift into a higher risk group in 29 patients (15%) with 0.5 mm reconstructions (Table 2).
The volume score was higher at 0.5 mm than at 3.0 mm reconstructions in 108 of 200 patients (mean ± SD: 180 ± 390 vs. 162 ± 375, mean difference 18, P < 0.01). In 16 cases, the volume score at 3.0 mm reconstructions was higher than at 0.5 mm reconstructions (mean ± SD: 156 ± 162 vs. 167 ± 172, a mean difference 11, P < 0.01).
All patients with a positive calcium score at 3.0 mm had positive calcium scores at 0.5 mm reconstructions as well.
Discussion
The main finding of the current study is that small amounts of coronary calcium are more sensitively depicted at thin 0.5 mm slice reconstructions than at standard 3.0 mm reconstructions. This resulted in a positive calcium score with 0.5 mm reconstructions in 21% of patients who had a zero calcium score at 3.0 mm reconstructions. Furthermore, calcium scores were significantly higher at 0.5 mm than at 3.0 mm reconstructions. The effect of using 0.5 mm instead of 3.0 mm reconstructions has more effect on the Agatston score than on the volume score.
In the present study, small and low attenuation calcifications were earlier depicted at 0.5 mm than at 3.0 mm reconstructions and higher calcium scores were found for thin-slice 0.5 mm reconstructions. The detection of small amounts of coronary calcium improves because of the smaller voxel size. Calcium score analysis software algorithms use certain attenuation thresholds for detection. If a voxel contains only part of a calcification or low attenuation calcium, the average attenuation value may fall below the detection threshold due to partial volume effect. With thin-slice reconstructions that imply smaller voxels, the chance of a voxel containing sufficient calcification attenuation for reaching the detection threshold increases, especially in small or low attenuation calcification [29]. Although image noise increases with thin-slice reconstructions (compared to thick-slice reconstructions from the same dataset), signal to noise ratio may not necessarily decrease. Thin-slice reconstructions lead to higher noise but also to a higher signal (due to the reduced partial volume effect). Therefore, the assumption that thin-slice reconstructions are associated with too much noise to distinguish image noise from calcifications may not be true, since not only image noise, but also the signal may increase.
More pronounced increase for Agatston scores than for volume scores is also explained by partial volume effect. The Agatston score is calculated as the product of the area of calcifications and a scaling factor based on the peak attenuation value within the calcified lesion [6]. In small voxels the detection threshold is reached earlier and the chance of a smaller voxel containing higher peak attenuation that has effect on the non-linear Agatston score increases. As such weighing factor is not applied in volume scores, these are less affected by slice thickness. The increase in Agatston score with 0.5 mm reconstructions would have led to an increase in risk group in 29 patients. However, risk stratification databases are based on 3.0 mm reconstructions. It is unknown if scores obtained with 0.5 mm datasets can be extrapolated to risk stratification schemes based on 3.0 mm slice reconstructions.
Our results are in line with previous studies that reported an increase in Agatston and volume scores with thinner slices [22, 29–31]. In one study, 3.0 mm calcium scores were compared to scores obtained with nonoverlapping 1.0 mm slices in 50 patients. In that study, the detection threshold for coronary calcium was increased from 130 to 350 HU to discriminate small calcified lesions from image noise. Small calcified lesions were found in 1.0 mm slice reconstructions in 4 of 27 patients with a zero score at 3.0 mm slice reconstructions. However, it was also reported that the increase in detection threshold lead to false-negative scoring results in 6 patients [29]. Similar results of improved calcium detection with thinner slices were found for other studies [9, 22, 31, 32]. However, these studies analysed the effect of thin-slice reconstructions predominantly in patients with positive calcium scores, whereas in the present study, the effect of thin-slice reconstructions focused on 100 patients with and 100 patients without coronary calcium at standard 3.0 mm reconstructions. One-fifth of the patients with a negative calcium score on 3.0 mm reconstructions were found to have coronary calcium on 0.5 mm reconstructions, suggesting that 0.5 mm reconstructions are more sensitive in depicting atherosclerotic coronary artery disease than traditional 3.0 mm reconstructions. Interestingly, in the present study, in seven patients the Agatson score was higher in 3.0 mm reconstructions than 0.5 mm reconstructions and in 16 patients a higher volume score was found in 3.0 mm reconstructions compared to 0.5 mm scores. This can be explained by 0.5 mm reconstructions with improved spatial resolution (smaller voxels) as compared to 3.0 mm reconstructions as well. Because of smaller voxels, partial volume effect decreases as calcium will only be calculated in voxels containing calcium but not in voxels not containing calcium, and may therefore result in decrease in calcium score by 0.5 mm reconstructions.
Furthermore, in contrary to a previous study [29], in the current study the traditional threshold value of 130 HU was used for both 3.0 and 0.5 mm calcium scores. This was done to maintain sensitivity for optimal coronary calcium detection as an increased attenuation threshold may lead to decreased sensitivity for small or low attenuation calcifications [29]. Moreover, clinical risk stratification is based on the threshold value of 130 HU as well, and this study therefore reflects changes for the investigated patient population under traditional scoring circumstances. Also, in the present study, volumetric acquisition was performed where the entire heart was imaged in a single gantry rotation. Using the same raw dataset for 3.0 and 0.5 mm reconstructions provided for optimal comparison between both slice thicknesses.
Although zero calcium scores are associated with a low risk for cardiovascular events in the following 2–5 years [33, 34], even zero calcium scores may not exclude luminal obstructive disease. In one study, 7% of patients with acute or long-term chest pain who had a zero calcium score were found having significant (>50% stenosis) coronary artery disease [35]. Especially in those patients who are young and presenting with acute coronary syndrome one cannot rely on a negative calcium score for ruling out obstructive coronary artery disease [36, 37]. A recent survey including nine outcome studies that had separate analysis of patients having zero calcium scores, reported in eight of these studies all-cause mortality on cardiovascular events of 0.4% after a follow-up period of 2.7–6.8 years. The one other study reported a percentage of 4.4%. In that study, 6.0 mm instead of 3.0 mm slices had been used for calcium score, which is another indicator that thicker slices may result in missing calcified lesions [13]. We found 21% of patients with coronary calcium only depicted on the 0.5 but not on 3.0 mm reconstructions. Increased sensitivity for coronary calcium detection by 0.5 mm slices may be expected to improve accuracy in ruling out coronary artery disease, especially in those patients having a zero calcium score at 3.0 mm reconstructions that remains a zero calcium score at 0.5 mm reconstructions. It should be noted that the same criteria for coronary artery calcium detection were used for 0.5 mm and for 3.0 mm reconstructions, of at least three face-connected voxels in the axial plane with an attenuation threshold of ≥130 HU.
Some study limitations are addressed. In this study we intended to compare calcium score by evaluating clinical acquisitions reconstructed at standard 3.0 mm slice thickness and 0.5 mm slice thickness. Although it was not our intention to provide a theoretical base and validation of calcium scores derived from thin-slice reconstructions and subsequent impact of image noise levels, our findings seem to be in agreement with some general considerations. With thin-slice reconstructions increased noise level was observed. As to avoid depicting image noise incorrectly as coronary calcium, we used direct lesion comparison between 3.0 and 0.5 mm reconstructions and used strict criteria for defining calcium spots; the size of the identified lesion had to be larger than spots of image noise on the same slice level and the lesion had to be present in at least two adjacent slices. Although a 320-detector-row volumetric scanner with single rotation full cardiac imaging was used for acquisition, thin-slice reconstructions may be obtained with other types of scanners as well, e.g. by using volumetric step-and-shoot acquisition techniques. It is not known what the effect of using calcium scores by thin-slice reconstructions would be in a large population by means of risk stratification and clinical consequences for individual patients. The findings of the present study are preliminary and based on a small sample size. Future outcome studies are needed to evaluate the clinical implications of thin-slice calcium scoring. Although slice thickness of 0.5 mm used in the study presented may differ from that of other vendors that may use 0.6 or 0.625 mm detector rows, 0.6 or 0.625 mm slice thickness would also be substantially smaller than the original 3.0 mm slices. Therefore, it is conceivable that increased detectability by thin-slice imaging of small or low attenuation calcifications accounts for 0.6 and 0.625 mm detector-row scanners as well.
In conclusion, with volumetric 320-detector row CT acquisitions, prospective ECG-triggered calcium scoring at 0.5 mm compared to 3.0 mm slice reconstructions leads to an increase in Agatston and volume scores and small amounts of coronary calcium are earlier depicted. This may be of special interest in symptomatic patients with zero calcium score by traditional 3.0 mm measures, where 0.5 mm reconstructions may help in better depicting or ruling out coronary artery disease.
References
Eggen DA, Strong JP, McGill HC Jr (1965) Coronary calcification. Relationship to clinically significant coronary lesions and race, sex, and topographic distribution. Circulation 32:948–955
Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT (2001) Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35, 246 adults. Am J Cardiol 87:1335–1339
Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy PF II, Peyser PA, Schwartz RS (2001) Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation 104:412–417
Wong ND, Hsu JC, Detrano RC, Diamond G, Eisenberg H, Gardin JM (2000) Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol 86:495–498
Carr JJ, Crouse JR III, Goff DC Jr, D’Agostino RB Jr, Peterson NP, Burke GL (2000) Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol 174:915–921
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832
Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P (1998) Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 208:807–814
Hoffmann U, Siebert U, Bull-Stewart A, Achenbach S, Ferencik M, Moselewski F, Brady TJ, Massaro JM, O’Donnell CJ (2006) Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort—consequences for progression studies. Eur J Radiol 57:396–402
Horiguchi J, Matsuura N, Yamamoto H, Hirai N, Kiguchi M, Fujioka C, Kitagawa T, Kohno N, Ito K (2008) Variability of repeated coronary artery calcium measurements by 1.25-mm- and 2.5-mm-thickness images on prospective electrocardiograph-triggered 64-slice CT. Eur Radiol 18:209–216
Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS (2007) Long-term prognosis associated with coronary calcification: observations from a registry of 25, 253 patients. J Am Coll Cardiol 49:1860–1870
Rumberger JA, Brundage BH, Rader DJ, Kondos G (1999) Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc 74:243–252
Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ Jr, Stein JH, Tracy CM, Vogel RA, Wesley DJ (2007) ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 expert consensus document on electron beam computed tomography). Circulation 115:402–426
Oudkerk M, Stillman AE, Halliburton SS, Kalender WA, Mohlenkamp S, McCollough CH, Vliegenthart R, Shaw LJ, Stanford W, Taylor AJ, van Ooijen PM, Wexler L, Raggi P (2008) Coronary artery calcium screening: current status and recommendations from the European society of cardiac radiology and north american society for cardiovascular imaging. Int J Cardiovasc Imaging 24:645–671
Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD (2000) Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol 36:1253–1260
Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ (2003) Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 228:826–833
Becker CR, Kleffel T, Crispin A, Knez A, Young J, Schoepf UJ, Haberl R, Reiser MF (2001) Coronary artery calcium measurement: agreement of multirow detector and electron beam CT. AJR Am J Roentgenol 176:1295–1298
Becker CR, Jakobs TF, Aydemir S, Becker A, Knez A, Schoepf UJ, Bruening R, Haberl R, Reiser MF (2000) Helical and single-slice conventional CT versus electron beam CT for the quantification of coronary artery calcification. AJR Am J Roentgenol 174:543–547
Kopp AF, Ohnesorge B, Becker C, Schroder S, Heuschmid M, Kuttner A, Kuzo R, Claussen CD (2002) Reproducibility and accuracy of coronary calcium measurements with multi-detector row versus electron-beam CT. Radiology 225:113–119
Ulzheimer S, Kalender WA (2003) Assessment of calcium scoring performance in cardiac computed tomography. Eur Radiol 13:484–497
Stanford W, Thompson BH, Burns TL, Heery SD, Burr MC (2004) Coronary artery calcium quantification at multi-detector row helical CT versus electron-beam CT. Radiology 230:397–402
Greuter MJ, Dijkstra H, Groen JM, Vliegenthart R, de Lange F, Renema WK, de Bock GH, Oudkerk M (2007) 64 slice MDCT generally underestimates coronary calcium scores as compared to EBT: a phantom study. Med Phys 34:3510–3519
Muhlenbruch G, Klotz E, Wildberger JE, Koos R, Das M, Niethammer M, Hohl C, Honnef D, Thomas C, Gunther RW, Mahnken AH (2007) The accuracy of 1- and 3-mm slices in coronary calcium scoring using multi-slice CT in vitro and in vivo. Eur Radiol 17:321–329
Yoon HC, Goldin JG, Greaser LE III, Sayre J, Fonarow GC (2000) Interscan variation in coronary artery calcium quantification in a large asymptomatic patient population. AJR Am J Roentgenol 174:803–809
Rybicki FJ, Otero HJ, Steigner ML, Vorobiof G, Nallamshetty L, Mitsouras D, Ersoy H, Mather RT, Judy PF, Cai T, Coyner K, Schultz K, Whitmore AG, Di Carli MF (2008) Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging 24:535–546
Dewey M, Zimmermann E, Deissenrieder F, Laule M, Dubel HP, Schlattmann P, Knebel F, Rutsch W, Hamm B (2009) Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation 120:867–875
Menzel HG, Schibilla H, Teunen D (2000) European guidelines on quality criteria for computed tomography. EUR 16262 EN. European Commission, Luxembourg
Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, Kondos G, Kronmal RA (2009) Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 53:345–352
Akram K, Voros S (2008) Absolute coronary artery calcium scores are superior to MESA percentile rank in predicting obstructive coronary artery disease. Int J Cardiovasc Imaging 24:743–749
Muhlenbruch G, Thomas C, Wildberger JE, Koos R, Das M, Hohl C, Katoh M, Gunther RW, Mahnken AH (2005) Effect of varying slice thickness on coronary calcium scoring with multislice computed tomography in vitro and in vivo. Invest Radiol 40:695–699
Horiguchi J, Nakanishi T, Ito K (2001) Quantification of coronary artery calcium using multidetector CT and a retrospective ECG-gating reconstruction algorithm. AJR Am J Roentgenol 177:1429–1435
Groen JM, Greuter MJ, Schmidt B, Suess C, Vliegenthart R, Oudkerk M (2007) The influence of heart rate, slice thickness, and calcification density on calcium scores using 64-slice multidetector computed tomography: a systematic phantom study. Invest Radiol 42:848–855
Vliegenthart R, Song B, Hofman A, Witteman JC, Oudkerk M (2003) Coronary calcification at electron-beam CT: effect of section thickness on calcium scoring in vitro and in vivo. Radiology 229:520–525
Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE (2006) Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American heart association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation 114:1761–1791
Cheng VY, Lepor NE, Madyoon H, Eshaghian S, Naraghi AL, Shah PK (2007) Presence and severity of noncalcified coronary plaque on 64-slice computed tomographic coronary angiography in patients with zero and low coronary artery calcium. Am J Cardiol 99:1183–1186
Rubinshtein R, Gaspar T, Halon DA, Goldstein J, Peled N, Lewis BS (2007) Prevalence and extent of obstructive coronary artery disease in patients with zero or low calcium score undergoing 64-slice cardiac multidetector computed tomography for evaluation of a chest pain syndrome. Am J Cardiol 99:472–475
Marwan M, Ropers D, Pflederer T, Daniel WG, Achenbach S (2009) Clinical characteristics of patients with obstructive coronary lesions in the absence of coronary calcification: an evaluation by coronary CT angiography. Heart 95:1056–1060
Henneman MM, Schuijf JD, Pundziute G, van Werkhoven JM, van der Wall EE, Jukema JW, Bax JJ (2008) Noninvasive evaluation with multislice computed tomography in suspected acute coronary syndrome: plaque morphology on multislice computed tomography versus coronary calcium score. J Am Coll Cardiol 52:216–222
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van der Bijl, N., de Bruin, P.W., Geleijns, J. et al. Assessment of coronary artery calcium by using volumetric 320-row multi-detector computed tomography: comparison of 0.5 mm with 3.0 mm slice reconstructions. Int J Cardiovasc Imaging 26, 473–482 (2010). https://doi.org/10.1007/s10554-010-9581-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-010-9581-8