Abstract
Aim
To detect the accuracy of real-time three-dimensional echocardiography (RT3DE) and two-dimensional echocardiography (2DE) for tricuspid annulus (TA) assessment compared with magnetic resonance imaging (MRI).
Methods
Thirty patients (mean age 34 ± 13 years, 60% males) in sinus rhythm were examined by MRI, RT3DE, and 2DE for TA assessment. End-diastolic and end-systolic TA diameter (TAD) and TA fractional shortening (TAFS) were measured by RT3DE, 2DE, and MRI. End-diastolic and end-systolic TA area (TAA) and TA fractional area changes (TAFAC) were measured by RT3DE and MRI. End-diastolic and end-systolic right ventricular (RV) volumes and ejection fraction (RV-EF) were measured by MRI.
Results
The TA was clearly delineated in all patients and visualized as an oval-shaped by RT3DE and MRI. There was a good correlation between TADMRI and TAD3D (r = 0.75, P = 0.001), while TAD2D was fairly correlated with TAD3D and TADMRI (r = 0.5, P = 0.01 for both). There were no significant differences between RT3DE and MRI in TAD, TAA, TAFS, and TAFAC measurements, while TAD2D and TAFS2D were significantly underestimated (P < 0.001). TAFS2D was not correlated with RV-EF, while TAFS3D and TAFAC3D were fairly correlated with RV-EF (r = 0.49, P = 0.01, and r = 0.47, P = 0.02 respectively).
Conclusion
RT3DE helps in accurate assessment of TA comparable to MRI and may have an important implication in the TV surgical decision-making processes. RT3DE analysis of TA function could be used as a marker of RV function.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Evaluation of tricuspid annulus (TA) continues to be a major problem in the surgical decision-making process due to its complex three-dimensional shape [1–3]. Accurate assessment of TA has many values in clinical application. For example, the decision of tricuspid valve repair may change due to discrepancy between pre-operative measurement of TA diameter (TAD) by two-dimensional transthoracic (or transesophageal) echocardiography (2DE) and direct surgical visualization [4]. Several studies described a strong correlation between TA motion and right ventricular (RV) function [5–8]. Analysis of TA velocity by tissue Doppler imaging has been found useful for RV functional assessment [9, 10]. In these studies, the assessment of TA motion relied on 2DE, M-mode and tissue Doppler recording. Since Real-time three-dimensional echocardiography (RT3DE) has become available for clinical practice, it is now possible to examine the TV more completely [11]. This study aimed to use RT3DE in evaluation of TA morphology, size and cyclic changes during the cardiac cycle. It also aimed to correlate TA function with RV function that was assessed by magnetic resonance imaging (MRI).
Subjects and methods
The study included 30 patients (mean age 34 ± 13 years, 60% were males) who were scheduled for routine MRI examination for evaluation of right ventricular function (10 patients with congenital heart disease, five with chronic pulmonary disease, five with multivalvular affection, and 10 normals). 2DE and RT3DE were performed at the same day of MRI examination after their informed consent for assessment of TA. The inclusion criteria for selection were good 2D image quality, sinus rhythm and mild to moderate tricuspid regurgitation.
MRI studies were performed with a 1.5 T MRI (General Electric, Milwaukee WI; Signa 1.5 T MRI) equipped with a four-element torso coil. A cardiac-triggered, steady-state, free-precession sequence (FIESTA; temporal resolution and repetition time of echo of 3.5 and 1.3 ms, respectively, flip angle of 45 degrees) was used for quantitative analysis. 10 cine short axis slices were acquired (slice thickness 10 mm, gap 0 mm) during a breath-hold period. Additional imaging parameters were a field of view of 320–380 mm and a matrix of 160 × 128. These series of high quality images encompassing the right ventricle (RV) produced a three-dimensional data set with sharp edge between the blood pool and myocardium. Quantitative analysis was performed using standardized software (MassPlus, Medis Inc., Leiden, NL). By this software, manual tracing was done for TA and RV endocardial border on all images (end-diastolic and end-systolic). The following measures were obtained at end-diastolic and end-systolic frame: (1) TA area (TAAMRI), (2) TADMRI, (3) RV volume, (4) RV ejection fraction (RV-EF) was calculated as (RV end-diastolic volume- RV end-systolic volume/RV end-diastolic volume) × 100%, (5) TA fractional shortening (TAFSMRI) defined as (end-diastolic TADMRI–end-systolic TADMRI)/end-diastolic TADMRI × 100% and (6) TA fractional area changes (TAFACMRI) defined as (end-diastolic TAAMRI–end-systolic TAAMRI)/end-diastolic TAAMRI × 100%.
2DE was done with a Sonos 7500 ultrasound system attached to a S3 transducer (Philips, Best, The Netherlands). The TV was imaged from apical 4-chamber view with the patient in the left lateral decubitus position. The following measures were obtained: (1) TAD2D was defined as the distance between the insertion sites of septal and anterior TV leaflets and obtained at an end-diastolic and end-systolic still frame and (2) TA fraction shortening (TAFS2D) defined as (end-diastolic TAD2D–end-systolic TAD2D)/end-diastolic TAD2D × 100%
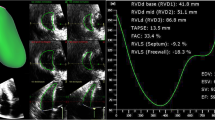
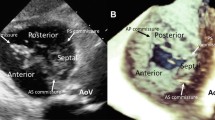
RT3DE was done with the same ultrasound system attached to X4 matrix array transducer capable of providing real-time B-mode images. A full volume 3D data set was collected within approximately 5–10 s of breath holding in full volume mode from an apical window. The 3D data set was transferred for off-line analysis with TomTec software (Unterschleissheim, Munich, Germany). Data were stored digitally and subsequently evaluated by two blinded observers (AMA, OIIS). Data analysis of 3D images was based on a 2D approach relying on images obtained initially from the apical view. The TA was sliced between two narrow lines to exclude other tissues on the 2D image leading to clarification of annulus by a 3D image. TA was viewed and traced manually from the atrial aspect and once this is completed the surface area was automatically calculated and could be visualized from different points of views. Manual modification was made to correct any inconsistence. The following RT3DE variables were obtained from both an end-diastolic and end-systolic still frame: (1) TA area (TAA3D), and (2) TAD3D defined as the widest TAD (see Fig. 1), Subsequently, TAFS3D and TA fractional area changes (TAFAC3D) (%) were calculated by the same formula used in 2DE.
Tricuspid annulus oval shape as seen by graphic representation (A), real-time three-dimensional echocardiography (B), magnetic resonance imaging (C), and tricuspid annulus with 2 lines inside, the larger is the TAD by real-time three-dimensional echocardiography and the smaller is the TAD by two-dimensional echocardiography with large difference in measurements
Statistical analysis
All data obtained by MRI, 2DE and RT3DE are presented as mean ± SD. A paired t-test and was performed for comparing means of variables. The level of significance was set to P < 0.05. A SPSS statistical package was used (SPSS, version 12.1, SPSS Inc, Chicago). Pearson’s coefficient was used for correlation between RT3DE and MRI data. Interobserver agreement for RT3DE measurements was assessed according to the Bland and Altman principle [12].
Results
Acquisition of RT3DE data was performed in all patients in a reasonable time (approximately 1 min for acquisition and 5 min for data analysis). The TA was clearly delineated in all patients. An oval-shaped TA (not circular) was visualized by RT3DE and MRI (Fig. 1). TAD3D was obtained with very good interobserver agreement (mean difference −0.4 ± 1.5 mm, agreement −3.4–2.6). There was a good correlation between TADMRI and TAD3D (R = 0.75, P = 0.001), while TAD2D was fairly correlated with TAD3D and TADMRI (R = 0.5, P = 0.01 for both). There were no significant differences between RT3DE and MRI in TAD, TAA, TAFS, and TAFAC measurements, while TAD2D and TAFS2D were significantly underestimated (P < 0.001) (Table 1). TAFS2D was not correlated with RV-EF, while TAFS3D and TAFAC3D were fairly correlated with RV-EF (r = 0.49, P = 0.01, and r = 0.47, P = 0.02 respectively).
Discussion
In the present study, the morphological and functional aspects of TA were assessed by MRI and RT3DE. The main findings of our study are (1) TA shape was not circular but oval, (2) TAD measurement by RT3DE is more accurate than by 2DE, and (3) TA function was fairly correlated with RV function.
TAD measurements have an important role in the TV surgical decision-making process not only for the selection of patients undergoing surgery, but also the type of surgical technique (valve plication or ring placement) [13–15]. Although 2DE is helpful to assess TV function and to detect TR severity it has important limitations in describing TV morphological details, such as TAD [14, 15]. RT3DE may yield more detailed anatomical description of TA morphology and function [16, 17]. In the present study, the TA was visualized well in all subjects allowing even measurements of its area. This is in accordance with Schnabel et al. [11] who reported well or at least sufficient TA visualization in over 90% of patients. The measurements of TAD by RT3DE and MRI showed good correlation without significant difference between both techniques. When TAD2D measurements were compared with the TAD3D and TADMRI measurements, 2D measurements were significantly underestimated. Analysis of TA motion by M-mode, 2DE and tissue Doppler imaging has been studied as a feasible marker for RV function reflecting the longitudinal RV shortening and lengthening. All these analyses described the physiological behaviour of TA plane systolic motion towards the apex along the RV long axis [5–10]. The TA plane systolic displacement is not influenced by its complex structure and asymmetrical shape [18] In the present study TA circumferential and horizontal motion along the RV short axis was assessed by RT3DE. TA motion along the RV short axis, which was fairly correlated with RV function that was assessed by MRI as a standard method for assessing RV ejection fraction. Fair and not good correlation may be explained by the meridional motion of the muscles and other structures of the RV inflow region. Furthermore, the myocytes are disposed longitudinally in the inflow region and more sensitive to meridional stress [19]. Despite this fair correlation, the summation of TA motion along both RV long and short axes increases the accuracy and correlation values for estimation of RV function [20].
Study limitation
The main limitation of this study is that RT3DE images more critically depend on image quality than 2DE images and the value of RT3DE should be assessed in a more non-selected (image quality). Due to the high cost of MRI, a small number of patients were included.
Conclusions
RT3DE helps in accurate assessment of TA comparable to MRI, while 2DE could not be relied on due to underestimation. This may have important implications in the TV surgical decision-making processes. RT3DE analysis of TA function could be used as a marker of RV function.
References
Silver MD, Lam JH, Ranganathan N, Wigle ED (1971) Morphology of the human tricuspid valve. Circulation 43:333–348
Yacoub MH, Cohn LH (2004) Novel approaches to cardiac valve repair: from structure to function: Part II. Circulation 109:1064–1072
McGrath LB, Gonzalez-Lavin L, Bailey BM, Grunkemeier GL, Fernandez J, Laub GW (1990) Tricuspid valve operations in 530 patients. Twenty-five-year assessment of early and late phase events. J Thorac Cardiovasc Surg 99:124–133
Colombo T, Russo C, Ciliberto GR, Lanfranconi M, Bruschi G, Agati S, Vitali E (2001) Tricuspid regurgitation secondary to mitral valve disease: tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc Surg 9:369–377
Alam M, Samad BA (1999) Detection of exercise-induced reversible right ventricular wall motion abnormalities using echocardiographic determined tricuspid annular motion. Am J Cardiol 83:103–5 A8
Shah AR, Grodman R, Salazar MF, Rehman NU, Coppola J, Braff R (2000) Assessment of acute right ventricular dysfunction induced by right coronary artery occlusion using echocardiographic atrioventricular plane displacement. Echocardiography 17:513–519
Therrien J, Henein MY, Li W, Somerville J, Rigby M (2000) Right ventricular long axis function in adults and children with Ebstein’s malformation. Int J Cardiol 73:243–249
Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, Gavazzi A, Tavazzi L (2000) Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol 85:837–842
Alam M, Wardell J, Andersson E, Samad BA, Nordlander R (1999) Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr 12:618–628
Alam M, Wardell J, Andersson E, Samad BA, Nordlander R (2000) Right ventricular function in patients with first inferior myocardial infarction: assessment by tricuspid annular motion and tricuspid annular velocity. Am Heart J 139:710–715
Schnabel R, Khaw AV, von Bardeleben RS, Strasser C, Kramm T, Meyer J, Mohr-Kahaly S (2005) Assessment of the tricuspid valve morphology by transthoracic real-time-3D-echocardiography. Echocardiography 22:15–23
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Goldman ME, Guarino T, Fuster V, Mindich B (1987) The necessity for tricuspid valve repair can be determined intraoperatively by two-dimensional echocardiography. J Thorac Cardiovasc Surg 94:542–550
De Simone R, Lange R, Tanzeem A, Gams E, Hagl S (1993) Adjustable tricuspid valve annuloplasty assisted by intraoperative transesophageal color Doppler echocardiography. Am J Cardiol 71:926–931
Dreyfus GD, Corbi PJ, Chan KM, Bahrami T (2005) Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg 79:127–32
Fukuda S, Saracino G, Matsumura Y, Daimon M, Tran H, greenberg NL, Hozumi T, Yoshikawa J, Thomas JD, Shiota T (2006) Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation. Circulation 114(suppl I):492–498
Anwar AM, Geleijnse ML, ten Cate FJ, Meijboom FJ Assessment of tricuspid valve annulus size, shape and function using real-time three-dimensional echocardiography. Interactive CardioVascular and Thoracic Surgery 2006; 10.1510/icvts.2006.132381
Ueti OM, Camargo EE, Ueti A de A, de Lima-Filho EC, Nogueira (2002) Assessment of right ventricular function with Doppler echocardiographic indices derived from tricuspid annular motion: comparison with radionuvleide angiography. Heart 88:244–248
Squara P, Journois D, Estagnasie P (1997) Elastic energy an index of right ventricular filling. Chest 11:351–358
Smith JL, Bolson EL, wang SP, Hubka M, Sheehan FH (2003) Three-dimensional assessment of two-dimensional technique for evaluation of right ventricular function by tricuspid annulus motion. Int J Cardiovasc Imaging 19(3):189–197
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Anwar, A.M., Soliman, O.I.I., Nemes, A. et al. Value of assessment of tricuspid annulus: real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging 23, 701–705 (2007). https://doi.org/10.1007/s10554-006-9206-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-006-9206-4