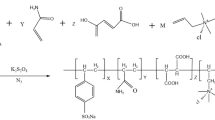
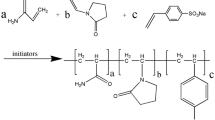
In this study, a desulfonated hybrid organic/inorganic fluid loss agent resistant to high temperatures is synthesized by using free-radical copolymerization. The fluid loss agent consists of the organic monomer acrylamide (AM), N-vinylpyrrolidone (NVP), dimethyl diallyl ammonium chloride (DMDAAC), and the inorganic monomer KH570 modified by nano-silica (M-SiO2). A field emission transmission electron microscope, an infrared spectrometer, and a thermogravimetric analyzer are used to examine the morphology and structure of the fluid loss agent. The results show that the inorganic nanoparticles and organic polymers are successfully grafted, and the resulting “core-shell” structure is connected by molecular chains. When 2% wt of the synthetic fluid loss additive E(AND-SiO2) is added to the base slurry, the normal-pressure fluid loss (FLAPI) and high-temperature, high-pressure fluid loss (FLHTHP) of the slurry are determined by the aging tests at 150, 160, 170, 180, 190, and 200°C for 16 hours. The results show that when the temperature is 180°C, FLAPI is 6.4 mL, FLHTHP is 28 mL, and temperature resistance is good. The biological toxicity and biodegradability tests show that the fluid loss control agent does not only effectively reduce the fluid loss, but also easily degrades, making it an environmentally-friendly treatment agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
Despite the new coronavirus disease outbreak, China’s oil and gas production still maintains a growing trend. Oil and gas resources are still the main forces driving forward the country’s development. The high-temperature water-based drilling fluid treatment agent for deep wells is one of the foundations for the development of oil and gas resources. Water-based drilling fluid loss additives are the main agents in oilfield treatments [1,2]. State-of-the-art high-temperature fluid loss control agents mainly include sulfomethyl lignite (SMC), sulfomethyl phenolic resin (SMP-1, SMP-2), sulfonated lignin sulfomethyl phenolic resin (SLSP), and other sulfonated products [3,4,5,6,7,8]. These treatment agents share one thing in common: they all contain a sulfonate group (-SO3-) in the carbon chain. Obviously, the sulfonate (-SO3-) ion is a necessary functional group for developing high-temperature treatment agents [9]. The Research Institute of Safety & Environment Technology of the China National Petroleum Corporation conducted a toxicity test on the drilling waste fluid of the sulfonation system and pointed out that the drilling fluid exceeds the national standard requirements in terms of color, biological toxicity, and chemical toxicity. This is attributed to the sulfonated phenolic resin and sulfonated asphalt added during the configuration. Research on environmentally-friendly desulfonated drilling fluids that meet standard engineering requirements would help to minimize the adverse impact of the drilling fluids on the environment and has important economic and social implications.
Inorganic modified nano-silica has a certain temperature-resistance function. Nanoparticles have good adsorption properties in amphoteric polymers [10], as they can block pores and reduce fluid loss [11]. Therefore, in this paper, based on the existing knowledge of inorganic and organic chemistry drilling fluid technology principles, high-temperature fluid loss mechanism, and temperature-resistance mechanism of the conventional treatment agents, a desulfonated hybrid organic/inorganic fluid loss agent E(AND-SiO2) resistant to high temperatures is developed. The fluid loss agent is a desulfonated water-based drilling fluid loss additive, which is harmless to the environment and is resistant to temperatures as high as 180°C and above.
2. Materials and methods
2.1 Materials
AM (99%, AR), NVP (99%, containing 100 ppm NaOH stabilizer), and DMDAAC (60% in water) are obtained from Aladdin Chemical Reagent Products. Methacryloxypropyltrimethoxysilane (KH570) modified nano-silica (20 nm, 99%) is purchased from Xianfeng Nano Reagent Company. Ammonium persulfate (98%), sodium bisulfite (98%), and sodium hydroxide are purchased from the domestic Cologne Chemical Reagent Company.
2.2 Methods
2.2.1. Synthesis of the E(AND-SiO2) grafted copolymer
In this research, we have synthesized the desulfonated hybrid organic/inorganic fluid loss agent that is resistant to high temperatures by the method of free radical copolymerization. The reagents AM (10 g), NVP (6 mL), and DMDAAC (8 g) are mixed in 50 mL deionized water, dissolved, and stirred. A certain amount of sodium hydroxide is used to adjust pH to 9. Then, M-SiO2 (0.5 g) is added into 50-mL deionized water and the ultrasonic method is used to fully disperse the modified nano-SiO2 for 25 min. Then, the solution is transferred to a 250 mL three-necked flask and heated to 70°C under the protection of nitrogen. At this moment, a certain amount of (NH4)2S2O8 is added to the solution in the three-necked flask and the solution is maintained for 7 h for the reaction with NaHSO3 to obtain a milky white viscous liquid. To prevent O2 from reducing the conversion rate during the experiment, nitrogen gas is used to discharge oxygen during the reaction. The reaction time is recorded. The obtained emulsion is dried and ground at 65°C to obtain a crude product.
2.2.2. Equation of the E(AND-SiO2) synthesis reaction
As shown in Fig. 2, the main chain of the molecule is formed by the C-C bonds. Compared with the main chain formed by heteroatoms, the C-C bond has higher bond energy and provides a certain chain rigidity and temperature resistance. The functional side chain should be formed by the combination of C-C, C-O, and C-N bonds with the main chain. These bonds have high bond energy and certain stability. They are not easy to break under the action of high temperature and ensure the performance of functional groups.
The morphology analysis diagram of the synthetic fluid loss additive E(AND-SiO2). a) Comparative test of the synthetic emulsion and unreacted complete emulsion; b) TEM image in ethanol solution at room temperature, magnified 100 nm; c) TEM image in ethanol solution at room temperature, magnified 200 nm
The influence of the synthetic fluid loss additive E(AND-SiO2) concentration on fluid loss performance. a) Histogram of filter loss before and after aging; b) the microscopic morphology of mud cake after aging at 180°C with the base slurry; c) the microscopic morphology of mud cake after aging at 180°C with the base slurry and 2% E(AND-SiO2)
3. Measurements
3.1. Structure characterization
Any substance is characterized by specific characteristics that differ from other substances. In this research, to determine the morphological characteristics, molecular structure, and thermal stability of E(AND-SiO2), we deploy the JEM-2100F field emission transmission electron microscope (TEM, Japan, Electronics Co, Ltd.) and the scanning electron microscope (SEM, FEI Czech Republic Sro). The Nicolet 6700 infrared spectrometer (FTIR, Thermo Scientific) is used to determine the position and intensity of the absorption peaks and to evaluate the functional groups contained in the synthetic fluid loss control agent exist. The Labsys EVO thermogravimetric analyzer (DTG, TGA, France Setaram) is used to analyze the thermal degradation of the synthetic fluid loss control agent.
3.2. Fluid loss characterization
In the context of drilling technology, the fluid loss parameter is usually used to describe the fluid loss of the drilling fluid. The loss meter notions of the normal temperature and pressure and the high temperature and pressure conditions are typically used to determine the amount of water loss under the specific conditions. The prepared fluid loss agent is added into 350 mL fresh water base pulp and aged at 180°C for 16 h. The water loss is measured with an interval of 30 min using a triplet normal temperature and medium pressure water loss meter and is registered as the normal temperature and pressure filtration loss (FLAPI). The less amount of water loss, the better the filtration effect. In general, the amount of API fluid loss should not exceed 5 mL. High-temperature and high-pressure fluid loss (FLHTHP) is measured by a high-temperature and high-pressure filter loss instrument. The prepared fluid loss control agent is added to the pre-configured fresh water base slurry and undergoes aging tests at different temperatures for 16 h, then, the top pressure is increased to 4 MPa. The lower pressure is adjusted to 0.5 MPa, and the bottom valve is opened to start the filtration loss test. Throughout the test, the pressure difference between the top pressure and the bottom pressure is maintained at 3.5 MPa, and the volume of the filtrate is recorded every 30 min. The less amount of water loss, the better the filtration effect. In general, FLHPHT should not exceed 20 mL. The quality of the mud cake formed in the experiment is a major indicator of fluid loss. If the mud cake is thin and dense, the fluid loss is small, whereas if the mud cake is thick and fluffy, the fluid loss is large. Therefore, the fluid loss and the quality of the mud cake are the key indicators of the fluid loss performance of a drilling fluid.
3.3. Environmental performance characterization
Currently, China has not established a separate environmental protection standard for drilling fluids, resulting in unclear directions for optimizing t h e environmental protection of the drilling fluid systems. According to the Integrated wastewater discharge standard (GB 8978-1996), evaluations of the drilling fluids are mainly focused on biological toxicity and biodegradability factors.
4. Results and discussions
4.1. Characterization of E(AND-SiO 2 )
Figure 3 shows the Fourier-transform infrared spectroscopy (FTIR) spectrum of E(AND-SiO2). The E(AND-SiO2) agent needs to be further purified before conducting the infrared analysis. The process requires dissolving the agent in water, adding ethanol, washing the precipitate several times, and then drying and grinding the powder product to avoid the presence of moisture and impurities.
The main characteristic peaks in the FTIR spectrum are the following: a low-intensity peak appears at 3440 cm–1, which is due to the symmetrical vibration of the C-H group and confirms the successful substitution reaction of DMDAAC on the SiO2 surface [12]; the peak at 2929 cm–1 is the stretching vibration peak of -CH2- in the main chain; the peak at 1650 cm–1 is characteristic of the C=O stretching vibration in the amide group (-CONH2-). The 1445 cm–1 peak is the C-N vibration absorption peak in DMDAAC, and the 1043 and 470 cm–1 peaks are the asymmetric stretching and bending vibrations of Si-O-Si. In addition, there is no characteristic absorption of C=C in the range of 1650-1640 cm–1. Therefore, the characteristic peak of E(AND-SiO2) indicates that all specific functional monomers are successfully incorporated during the polymerization process. The functional monomer amide group in the synthesized copolymer E(AND-SiO2) as a non-ionic adsorption group has good adsorption stability. The macromolecular group adjacent to the amide group will hinder the amide group that has not been hydrolyzed. The effect prevents it from being hydrolyzed, so it has a better high-temperature resistance [13].
The TEM image provides the appearance and internal structure of the E(AND-SiO2) fluid loss additive. The result is shown in Fig. 4.
Figure 4 (a) shows the microemulsion of the E(AND-SiO2) fluid loss agent. It can be seen that the white emulsion is evenly dispersed and no precipitation appears after long-term storage for 3 days. The unreacted solution is maintained for 1 day and the solution is obviously layered. Figures 4 (b) and (c) demonstrate the morphology of the agent after being dried, crushed, and dispersed in ethanol solution. The image shows regular spherical shapes and the shapes are evenly distributed, whereas the molecular chains are formed. A “core-shell” structure is formed where the molecular chains are linked, whereas there are obvious branches on the main chain.
Thermogravimetric analysis is currently the most commonly used thermal stability test method. The result is shown in Fig. 5.
Figure 5 shows that the TG thermal analysis curve of the fluid loss agent E(AND-SiO2) is divided into three stages. In the first stage, the fluid loss agent loses less heat between 30-269°C, which may be caused by the volatilization of a small amount of water molecules in the sample. The specific reason may be that the synthesized polymer contains a lot of hydrophilic groups that can easily absorb moisture in the air and make the sample damp [14]. In the second stage, the fluid loss agent loses heat between 269-337°C and the heat loss tends to decrease linearly. This process is due to the decomposition and volatilization of the amide group in the molecular structure. In the third stage, the thermal analysis curve of the fluid loss additive tends to be flat after 337°C, indicating that the main chain breaks, thermal degradation occurs, and the structure of the copolymer is destroyed. This shows that when the temperature is lower than 269°C, the fluid loss additive has better thermal stability and the functional groups do not become invalid due to thermal degradation below this temperature.
4.2. Influence of E(AND-SiO 2 ) concentration on fluid loss performance
Base slurry configuration is as follows: 4% bentonite and 5% Na2CO3 are added to 1L of water, stirred for 30 min, and maintained at room temperature for 24 h to obtain fresh water base slurry.
Then, different quantities of the E(AND-SiO2) fluid loss agent are added to the base slurry. To test the fluid loss, the base slurry is subjected to aging at 180°C for 16 h. The test results are shown in Fig. 6.
Figure 6 shows that as the concentration of the fluid loss agent E(AND-SiO2) increases, the fluid losses before and after aging gradually decrease. When the dosage is 3.0%, the FLAPI before aging decreases from 39 to 4 mL, the FLAPI after aging decreases from 54 to 6 mL, and the FLHTHP after aging decreases from 72 to 21 mL. The mud cake formed by the base slurry aging at 180°C is thick and fluffy (the SEM image shows more voids). Furthermore, the mud cake formed by adding 2% E(AND-SiO2) is thin and dense (as shown by the SEM image). This shows that the filtrate reducer has an obvious filtering effect. It is proved that the synthetic E(AND-SiO2) plays the adsorption and bridging effect. When the agent is adsorbed on the clay particles, the degree of hydration is increased, the hydration film of the particles becomes thicker, and the flocculation of the clay particles is prevented. The filter cake has a low permeability. In addition, the synthesized polymer molecular chains are more likely to bridge with each other forming a space grid structure, which can further reduce the fluid loss [15].
4.3. The influence of temperature on the performance of E(AND-SiO 2 ) fluid loss agent
Based on the above results, 2% E(AND-SiO2) fluid loss additive is added to the fresh water base slurry. Then, FLAPI and FLHTHP are tested at 150, 160, 170, 180, 190, and 200°C for 16 hours. The test results are shown in Fig. 7.
As shown in Table 1 and Fig. 7, with increase in the aging temperature, the viscosity of the base slurry gradually decreases, whereas the fluid loss increases. However, even after aging tests at 180°C, the base slurry still has good rheological properties. Furthermore, FLAPI and FLHTHP are maintained at relatively low levels, namely 6.4 and 28 mL, respectively. When the aging temperature increases to 190°C, the FLAPI significantly increases, indicating that the temperature resistance limit of the synthesized product is 180°C. The hydrophilic groups are adsorbed on the clay surface forming a dense membrane structure. The electrostatic interaction between the quaternary amine groups and the clay particles is strong, which makes the polymer difficult to desorb under high-temperature conditions. The polymer can effectively prevent high-temperature dehydration of the clay. Inorganic modified nano-silica plays a certain temperature-resistance role in the agent. AM, DMDAAC, and NVP have high-temperature resistance groups, that further improve the high-temperature stability of the molecular chain. Therefore, the fluid loss agent E(AND-SiO2) has good resistance to high temperatures.
4.4. The influence of calcium salt on the fluid loss performance of E(AND-SiO 2 ) fluid loss agent
When the fluid loss control agent in the drilling fluid does not meet the requirements of the salt and calcium resistance under high-temperature conditions, the fluid loss will increase and the rheological properties will deteriorate. To test the anti-temperature, anti-salt, and anti-calcium properties of the fluid loss agent E(AND-SiO2) under HTHP, the fresh water based mud is mixed with 2% E(AND-SiO2), different amounts of NaCl (0, 3%, 6%, 9%,12%), and CaCl2 (0, 0.2%, 0.4%, 0.5%). Then, the FLAPI and FLHTHP values are measured before and after aging at 180°C for 16 h. The experimental results are shown in Fig. 8.
Figure 8 shows that the fresh water base slurry contains 2% E(AND-SiO2) fluid loss agent. After fully stirring and adding different concentrations of NaCl and different concentrations of CaCl2, the FLAPI and FLHTHP values both increase, but still remain within a certain range, indicating that E(AND-SiO2) has a good salt resistance (NaCl) and calcium resistance (0.5% CaCl2) under high temperatures (180°C).
5. Determination of the water-based drilling fluid system formula and the performance evaluation
To investigate the effect of E(AND-SiO2) fluid loss agent on the rheological properties, fluid loss, and temperature resistance of the system, we compare the drilling fluid system 1 without E(AND-SiO2) fluid loss agent and the fluid loss agent system 2 containing 2% E(AND-SiO2). The rheological fluid loss properties of the two systems are compared and analyzed.
№1: 4% base slurry + soil 5% Na2CO3 + 0.5% coating agent + 0% E(AND-SiO2) fluid loss agent (laboratory prepared) + 1% lubricant + 64% weighting agent;
№2: 4% base slurry + soil 5% Na2CO3 + 0.5% coating agent + 2% E(AND-SiO2) fluid loss agent (laboratory prepared) + 1% lubricant + 64% weighting agent.
5.1. Rheological fluid loss of the system
After configuring the appropriate amounts of the above water-based drilling fluid systems 1 and 2, the rheological properties and the fluid loss values before and after high-temperature 180°C aging are tested. The results are shown in Table 2.
Table 2 demonstrates that the addition of the fluid loss agent E(AND-SiO2) increases the viscosity and shear strength of the drilling fluid system and the fluid loss before and after aging decreases. This shows that the E(AND-SiO2) agent has good compatibility with the treatment agents in the system, has little effect on the rheological properties of the drilling fluid, and can effectively decrease fluid loss.
5.2. Analysis of temperature resistance
Based on the above temperature-resistant water-based drilling fluid system formulations 1 and 2, the aging tests are conducted at 150, 160, 170, 180, 190, and 200°C for 16 h to determine FLAPI and FLHTHP. The test results are shown in Fig. 9.
It can be seen that the FLAPI and FLHTHP of the water-based drilling fluid system 2 are lower than those of the system 1. The addition of the fluid loss agent E(AND-SiO2) decreases the fluid loss of the drilling fluid system. After aging at 180°C, the FLAPI and FLHTHP are maintained at low levels, namely 4.8 and 14 mL, respectively. This shows that the E(AND-SiO2) fluid loss agent provides a better temperature resistance.
6. Environmental performance evaluation
Environmentally-friendly drilling fluids do not produce hazardous waste, are non-toxic to humans and animals, do not inhibit plant growth and development, and are easily degraded [16, 17]. Therefore, according to the environmental performance evaluation index, the researchers focused on assessing the biological toxicity and biodegradability of the drilling fluid system.
6.1. Biological toxicity test
The drilling fluid mixture is composed of water and various chemical additives, and its biological toxicity mainly comes from drilling fluid additives. Commonly used biological toxicity detection methods include the use of mysis and luminescent bacteria [18, 19]. The method involving luminescent bacteria has a more rapid response, lower cost, and higher efficiency, and is more environmentally friendly. Therefore, the researchers have selected this method for assessing the biological toxicity of the drilling fluid system. The EC50 is used to indicate biological toxicity, which is the concentration at which the luminous intensity of the luminescent bacteria decreases by 50%. Studies have shown that the lower the toxicity of the drilling fluid and the lower the concentration, the higher the luminescence intensity of the luminescent bacteria. Therefore, the luminescence intensity can be used to characterize the biological toxicity of the drilling fluid. The evaluation criteria are shown in Table 3.
The biotoxicity evaluation of the E(AND-SiO2) fluid loss agent and the drilling fluid system 2 are performed using the luminescent bacteria method. The results are shown in Table 4.
Comparing Tables 3 and4 and Fig. 10, we can conclude that the EC50 values of the synthetic E(AND-SiO2) fluid loss agent and the drilling fluid system 2 are both higher than 104, which indicates the non-toxic fluid loss agent. The biological toxicity EC50 values of the existing desulfurization drilling fluids are generally between 1000-10000 [20]. It can be seen that the environmental performance effect of the fluid loss control agent and the developed drilling fluid system synthesized in this article has been improved.
6.2. Biodegradability tests
The focus of the biodegradability test of the drilling fluid is mainly to assess the biodegradability of the treatment agents added to the drilling fluid system. State-of-the-art evaluation methods include BOD5/CODCr (BOD refers to the biochemical oxygen consumption, while COD refers to the chemical oxygen consumption) [21, 22]. The ratio BOD5/CODCr defines the biodegradability of organic matter. The higher the ratio, the easier the organic matter biodegrades. The evaluation criteria are shown in Table 5.
The testing results of the E(AND-SiO2) fluid loss agent and the water-based drilling fluid system 2 are shown in Table 6.
Tables 5 and 6 show that the BOD5/CODCr ratio of the E(AND-SiO2) fluid loss additive is 0.49, indicating that it can easily biodegrade, as the index is higher than 0.45. Moreover, the BOD5/CODCr ratio of the water-based drilling fluid system 2 is 0.51. In summary, the fluid loss reduction agent is easy to biodegrade when added to the water-based drilling fluid system.
7. Conclusions
The organic/inorganic hybrid graft copolymer E(AND-SiO2) fluid loss control agent is synthesized by the free radical copolymerization method. The amount of 2% fluid loss agent E(AND-SiO2) is added to the base slurry. Even after the aging tests at 180°C, the base slurry retains its good rheological properties, and the FLAPI and FLHTHP are maintained at low levels, namely 6.4 and 28 mL, respectively. At the same time, the fluid loss control agent also has good resistance/tolerance to the salt (saturated NaCl) and calcium (0.5% CaCl2) concentrations.
To investigate the effect of the E(AND-SiO2) fluid loss agent on the system rheological properties, fluid loss, and temperature resistance, system 1 without E(AND-SiO2) and system 2 containing 2% E(AND-SiO2) are configured, and the rheological fluid loss performance of the two systems is compared and analyzed. The results show that the addition of the fluid loss agent E(ANDSiO2) increases the viscosity and the shear strength of the drilling fluid system. After high-temperature aging tests at 180°C, the FLAPI value is 4.8 mL, whereas the FLHTHP water loss is 14 mL. This shows that the E(AND-SiO2) fluid loss agent has better resistance to temperature.
Through biological toxicity and biodegradability assessments, it was shown that the fluid loss agent E(AND-SiO2) does not only effectively reduce the fluid loss, but can also easily degrade, making it an environmentally-friendly treatment agent.
References
H. Mao, W. Wang, Y. Ma, and Y. Huang, “Synthesis, characterization, and properties of an anionic polymer for water-based drilling fluid as an anti-high temperature and anti-salt contamination fluid loss control additive,” Pol. Bull., 78(5), 2484-2503 (2021).
Y. Li, J. Huang, and G. Yang, “Preparation of amphoteric phenol-formaldehyde resin as filtration loss reducer XNSMP-III for water-base drilling fluids,” Oilfield Chem., 26(4), 351-353 (2009).
S. M. Wang, J. Zhen, and Y. J. Qiao, “Synthesis and performance of sulfomethyl lignite potassium humate-acrylonitrile graft polymer anti-high temperature and salt-resistant fluid loss agent,” Humic Acid., 3, 23-27 (2001).
X. Bai, Y. Yang, D. Xiao, X. Pu, and X. Wang, “Synthesis, characterization, and performance evaluation of the AM/AMPS/DMDAAC/SSS quadripolymer as a fluid loss additive for water-based drilling fluid.” J. Appl. Pol. Sci., 132(14),41762 (2015).
A. C. Perricone, D. P. Enright, and J. M. Lucas, “Vinyl sulfonate copolymers for high-temperature filtration control of water-based muds,” SPE Drill. Eng., 1(5), 358-364 (1986).
C. Jie, Y. Tan, Y. Che, and M. Qiang, “Synthesis of copolymer of acrylamide with sodium vinylsulfonate and its thermal stability in solution,” J. Pol. Res., 18(2), 171-178 (2011).
L. Yang, G. Jiang, Y. Shi, X. Lin, and X. Yang, “Application of ionic liquid to a high-performance calcium-resistant additive for filtration control of bentonite/water-based drilling fluids,” J. Mater. Sci., 52(11), 6362-6375 (2017).
J. N. Yan, Drilling Fluid Technology, Petroleum University Press, Beijing, China (2013).
J. Ma, Y. An, and P. Yu, “Core-shell structure acrylamide copolymer grafted on nano-silica surface as an anti-calcium and anti-temperature fluid loss agent,” J. Mater. Sci., 54(7), 5927-5941 (2019).
P. A. Williams, R. Harrop, and I. D. Robb, “Adsorption of an amphoteric polymer on silica and its effect on dispersion stability,” J. Coll. Interface Sci., 102(2), 548-556 (1984)
T. Sensoy, M. E. Chenevert, and M. Sharma, “Minimizing water invasion in shales using nanoparticles,” SPE Annual Technical Conference and Exhibition 2009, New Orleans, LA, United States (2009).
H. Tao, Study on Synthesis, Characterization and Action Mechanism of High Temperature and Salt Resistance Calcium Water-based Filtrate Reducer for Drilling Fluid, Southwest Petroleum University, China (2012).
E. Pefferkorn, L. Nabzar, and A. Carroy, “Adsorption of polyacrylamide to Na kaolinite: Correlation between clay structure and surface properties,” J. Coll. Interface Sci., 106(1), 94-103 (1985).
C. Mai, “Synthesis of anti-high temperature fluid loss agent and preparation of drilling fluid system,” Chin. Pet. Chem. Stand. Qual., 40(18), 136-137(2020).
X. Yan, Study on the Synthesis and Performance of a Temperature-resistant and Salt-water-based Fluid Loss Control Agent for Drilling Fluids, China University of Petroleum, China (2019).
T. Abo, H. Husam, et al., “Experimental investigation of environmentally friendly drilling fluid additives (mandarin peels powder) to substitute the conventional chemicals used in water-based drilling fluid,” J. Pet. Explor. Prod. Technol., 10(2), 407-417 (2019).
S. Davoodi, A. Ramazani, V. Rukavishnikov, et al., “Insights into application of acorn shell powder in drilling fluid as environmentally friendly additive: filtration and rheology,” Int. J. Environ. Sci. Technol., 18(4), 835-848 (2021).
R. Anne, U. Mikko, K. Nitesh, et al., “Luminescent bacteria-based sensing method for methylmercury specific determination,” Anal. Bioanal. Chem., 400(4), 1041-1049 (2011).
D. Wang, Y. Gao, Z. Lin, Z. Yao, and W. Zhang, “The joint effects on photobacterium phosphoreum of metal oxide nanoparticles and their most likely coexisting chemicals in the environment,” Aquatic Toxicol., 154, 200-206 (2014).
J. Zhang, D. Zhou, Y. Liu, J. Chen, L. Chen, and H. Gu, “Research on desulfonated environmentally friendly drilling fluid,” Drill. Prod. Technol., 43(A1), 85-90 (2020).
W. Zhang, S. Isabelle, A. Abdeltif, and F. Florence, “Electrochemical processes coupled to a biological treatment for the removal of iodinated X-ray contrast media compounds,” Front. Chem., 8, 646 (2020).
Y. Lou, W. He, E. Verlato, M. Musiani, et al., “Ni-coated graphite felt modified with Ag nanoparticles: A new electrode material for electro-reductive dichlorination,” J. Electroanal. Chem., 849, 113357 (2019).
Acknowledgments
This work is supported by the Science and Technology Cooperation Project of the China National Petroleum Corporation - Southwest Petroleum University (CNPC-SWPU) Innovation Alliance (No.2020CX040201, 2020CX040102) and the National Science and Technology major projects (No. 2016ZX05022001-002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 1, pp. 166–171 January – February, 2022.
Rights and permissions
About this article
Cite this article
Su, J., Zhang, A., Zuo, F. et al. Study on the Preparation and System of Desulfonated Anti-High Temperature Fluid Loss Agent. Chem Technol Fuels Oils 58, 169–180 (2022). https://doi.org/10.1007/s10553-022-01364-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-022-01364-w