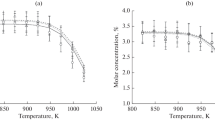
Investigations on the thermal contact pyrolysis of propane were undertaken in order to compare the equilibrium yields of the pyrolysis products and the real kinetic data. The method of minimization of the energy of the system, an advantage of which is the need to know only the initial and final composition of the components of the reaction system, was used to determine the yields of the pyrolysis products. It was shown that the yields of the pyrolysis products can be predicted by means of calculated equilibrium thermodynamic yields. It was shown that the accuracy of the calculated data depends on the pyrogas components formed during pyrolysis and specified at the beginning of the calculation. It was found that the real concentrations of the pyrolysis products in the pyro gas can be both higher and lower than the calculated concentrations. This will only indicate attainment or nonattainment of the equilibrium state of the system in the course of the process. The method can be used for the pyrolysis of other hydrocarbons with the aim of preliminary assessment of the maximum possible yields of the products during the process.





Similar content being viewed by others
References
A. R. Bikchurina, I. V. Tsivunina, Vestnik Kazanskogo Tekhnologicheskogo Universiteta, 18, No. 9, 137–139 (2015).
S. P. Gorislavets, D. N. Tmenov, V. I. Maiorov, Pyrolysis of Hydrocarbon Feedstock [in Russian], Naukova Dumka, Kiev (1977), 309 pp.
T. N. Mukhina, N. L. Barabarnov, S. E. Babash, et al., Pyrolysis of Hydrocarbon Feedstock, Khimiya, Moscow (1987), 240 pp.
V. V. Shinkarev, V. M. Khanaev, E. A. Solov’ev, Kinetika i Kataliz, 55, No. 1, 90–95 (2014).
N. M. Pogosyan, M. Dzh. Pogosyan, S. D. Arsent’ev, Neftekhimiya, 56, No. 6, 612–616 (2016).
A. I. Klement’ev, Izvestiya Vuzov. Neft’ i Gaz, No. 6, 100–102 (2014).
Yu. A. Aleksandrov, V. M. Shekunova, I. I. Didepkulova, et al., Zhurn. Obshch. Khim., 78, No. 10, 1662–1664 (2008).
N. V. Korzun, Yu. Yu. Lazarev, T. A. Sukhodolina, No. 1, 96–100 (2001).
P. O. Gus’kov, F. G. Zhagfarov, A. L. Lapidus, Gazokhimiya, No. 2, 20–24 (2011).
F. R. Murtazin, Neftepererabotka i Neftepererabotka, No. 8, 45–47 (2007).
R. G. Khasanov, F. R. Murtazin, Chemistry and Technology of Fuels and Oils, No. 3, 341–346 (2020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 3, pp. 25–27, May–June, 2021.
Rights and permissions
About this article
Cite this article
Khasanov, R.G., Zakharov, N.M. & Gaziev, R.R. Some Principles of Thermal Contact Pyrolysis of Propane. Chem Technol Fuels Oils 57, 451–455 (2021). https://doi.org/10.1007/s10553-021-01264-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-021-01264-5