Abstract
Purpose
Studies suggest that patients with type two diabetes mellitus (T2D) may be at increased risk of post-colonoscopy colorectal cancer (PCCRC). We investigated clinical and molecular characteristics and survival of T2D patients with PCCRC to elucidate how T2D-related PCCRC may arise.
Methods
We identified T2D patients with colorectal cancer (CRC) from 1995 to 2015 and computed prevalence ratios (PRs) comparing clinical and molecular characteristics of CRC in T2D patients with PCCRC vs. in T2D patients with colonoscopy-detected CRC (dCRC). We also followed T2D patients from the diagnosis of PCCRC/dCRC until death, emigration, or study end and compared mortality using Cox-proportional hazards regression models adjusted for sex, age, year of CRC diagnosis, and CRC stage.
Results
Compared with dCRC, PCCRC was associated with a higher prevalence of proximal CRCs (54% vs. 40%; PR: 1.43, 95% confidence interval [CI] 1.27–1.62) in T2D patients. We found no difference between PCCRC vs. dCRC for CRC stage, histology, and mismatch repair status. The proportion of CRCs that could be categorized as PCCRC decreased over time. Within one year after CRC, 63% of PCCRC vs. 78% of dCRC patients were alive (hazard ratio [HR] 1.85 [95% CI 1.47–2.31]). Within five years after CRC, 44% of PCCRC vs. 54% of dCRC patients were still alive (HR 1.44 [95% CI 1.11–1.87]).
Conclusion
The increased prevalence of proximally located PCCRCs and the poorer survival may suggest overlooked colorectal lesions as a predominant explanation for T2D-related PCCRC, although altered tumor progression cannot be ruled out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Endoscopy Organization (WEO), post-colonoscopy colorectal cancer (PCCRC) is defined as colorectal cancer (CRC) diagnosed >6 months and up to 36 months after a colonoscopy negative for CRC [1]. It is assumed that the majority of PCCRCs occur due to precancerous lesions that were missed or incompletely removed during the colonoscopy procedure [1]. Only a small proportion is thought to arise from different tumor biology underlying CRC development [1,2,3]. Existing literature indicates an increased proportion of PCCRC in patients with specific diseases such as diverticular disease and inflammatory bowel disease, with PCCRCs accounting for up to 50% of all CRCs diagnosed among these patients [1, 4, 5]. In addition, three studies have suggested an association between type two diabetes (T2D) and PCCRC [6,7,8]. Hence Laish et al. reported that 31% of all PCCRC patients had prevalent diabetes mellitus, while Suceveanu at al. found PCCRC and adenomas to be more frequent among patients with T2D than among patients without T2D [6, 7]. Finally, Troelsen et al. suggested an increased T2D-related risk of PCCRC [8]. T2D patients may achieve poorer bowel preparation for colonoscopy than non-T2D patients due to functional impairment of gastrointestinal motility. While such impairment could complicate colonoscopy and increase the risk of overlooking CRC and its precursors, evidence remains limited [9, 10]. Existing literature also indicates that the prevalence of proximally located polyps is higher in T2D patients, a localization associated with an increased risk of missed polyps and thus PCCRC [11]. At the same time, the consequences of long-term T2D (e.g., hyperinsulinemia, hyperglycemia, and microbiota changes) may affect the carcinogenenic processes leading to CRC growth in the interval between two colonoscopies [12, 13]. These findings combined could lead to a different ratio between T2D-related PCCRCs that arise through missed precursors and different or cancer development than for non-diabetic patients, but evidence is missing. The issue is compounded by the increasing global incidence of T2D [14, 15] and the introduction of CRC screening programs with the same inclusion criteria for T2D patients and non-diabetic patients. We therefore conducted the present study to examine characteristics and survival of T2D-related PCCRC to elucidate potential explanations for T2D-related PCCRC.
Methods
Setting and data sources
This nationwide study of T2D prevalence and prognosis was designed to use prospectively collected data from Danish national health databases during the January 1, 1995–December 31, 2015 period. The study was conducted within the setting of universal, tax-funded health care provided to all legal residents of Denmark by the National Health Service [16]. All persons residing in Denmark are registered in the Danish Civil Registration System (CRS) and assigned a unique personal identification number, which enables accurate and individual-level data linkage among all Danish health and administrative registries [16,17,18]. All registries and codes used in the analyses are described in Supplementary Tables 6-8.
The study was reported to the Danish Data Protection Agency by Aarhus University (record No. 2016-051-000001/1671).
Colorectal cancer patients
Patients with a first-time CRC diagnosis were identified from the Danish Cancer Registry (DCR). The DCR also provided information on CRC diagnosis date, location, and stage at diagnosis. CRCs were categorized using the following anatomic locations: proximal to the splenic flexure, distal to the splenic flexure, rectal, or unspecified/more than one site. CRCs also were categorized by stage at diagnosis according to the TNM classification: localized, regional, metastatic, or unknown.
Information on tumor histology at diagnosis and mismatch repair status (MMR) (when available) was obtained from the Danish National Pathology Registry (DPR). We categorized CRC histology as adenocarcinoma, polyp adenocarcinoma (i.e., invasive growth within an endoscopically well-defined polyp), mucinous carcinoma, and signet cell cancer. CRCs lacking a histological diagnosis in the DPR were categorized as “not histologically verified”. CRCs with an available test result for MMR status (N=61.8%) demonstrating nuclear absence of at least one of four MMR proteins (MLH1, MSH2, MSH6, or PMS2) were categorized as MMR-deficient. CRCs with a test demonstrating normal MMR status were categorized as MMR-proficient.
In addition, we categorized CRCs according to surgeries performed, based on surgical codes from the Danish National Patient Registry (DNPR) recorded within 90 days following a CRC diagnosis.
Detected and post-colonoscopy colorectal cancers
We linked the CRC cohort to the DNPR to obtain information on colonoscopies performed within three years before the CRC diagnosis. By design, the study excluded all CRCs diagnosed in patients who did not undergo a colonoscopy during this prior three-year period. All included CRCs were categorized as either PCCRCs or CRC detected during colonoscopy (dCRCs). We defined PCCRCs in accordance with World Endoscopy (WEO) recommendations, i.e., CRC diagnosed >6 to 36 months after a colonoscopy that found no CRC [1] and dCRCs as CRC diagnosed within 6 months after a colonoscopy.
Assessment of diabetes mellitus
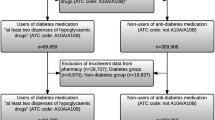
We identified PCCRC and dCRC patients who also had a T2D diagnosis from the DNPR and the Danish National Health Service Prescription Database (DNHSPD). All patients who redeemed a prescription for a glucose-lowering drug and/or had a diagnosis of diabetes mellitus recorded before or within 90 days after the colonoscopy that failed to detect the CRC/precancerous lesion (for PCCRCs) or that detected the CRC (for dCRCs) were considered to be T2D patients. According to a previously reported algorithm [19], patients younger than 30 years of age, who had a registered diagnosis of diabetes or redeemed a prescription for a glucose-lowering drug before or within 90 days of the colonoscopy that either failed to detect or detected the CRC were considered to be have type 1 diabetes and were excluded from the study. We categorized T2D patients according to T2D duration, measured in years from first-time prescription of a glucose-lowering drug or a first-time T2D diagnosis until CRC diagnosis (< 1 year, 1–5 years, 6–10 years, and > 10 years). Patients without T2D were excluded.
Assessment of covariates
We collected data from the DPR on colorectal polyps detected before the diagnosis of CRC. We included sessile serrate lesions and conventional adenomas. If no information concerning polyps was found in the DPR, we searched the DNPR using codes for endoscopically performed polypectomies recorded before the CRC diagnosis. We collected information on comorbidities from all records available in the DNPR from 1977 until the date of CRC diagnosis. To characterize the study cohort, we obtained discharge diagnoses of chronic obstructive pulmonary disease, cardiovascular diseases including atrial fibrillation/flutter, renal disease, alcohol-related diseases (i.e., alcohol-related psychosis, alcoholism, alcohol intoxication, chronic alcoholic pancreatitis, alcoholic liver cirrhosis, steatosis, brain degenerative changes caused by alcohol, alcoholic myopathy, and alcoholic gastritis), and obesity. We used Charlson Comorbidity Index (CCI) scores as a measure of the burden of comorbidity. The CCI scoring system allocates from one to six points to a range of diseases, as components of a summed aggregate score. Patients were categorized into three subgroups according to their calculated CCI score: low (no comorbidities) = CCI score of 0; medium = CCI score of 1–2; or high = CCI score of 3 or more (Supplementary Table S8).
Statistics
We calculated prevalence proportions (PPs) and prevalence ratios (PRs) comparing characteristics of PCCRCs vs. dCRCs among T2D patients. The robust Poisson method was used to calculate PRs and associated 95% confidence intervals (CIs) [20].
We also followed the cohort of T2D patients from the date of CRC diagnosis until first occurrence of all-cause death, emigration, or the administrative end of follow-up (December 31, 2015). Survival probabilities were computed using the Kaplan-Meier technique. We used Cox-proportional hazard regression models to compute hazard ratios (HRs). No violation to the proportional hazard assumption was found. Crude and adjusted HRs and associated 95% CIs were used as an estimate of the mortality rate ratio (MRR) comparing PCCRC in T2D patients with dCRC in T2D patients. We constructed two separate regression models adjusted for potential confounders of the association between PCCRC and death. The first model included age, sex, and year of CRC diagnosis. To include the effect of more advanced CRC stage in PCCRC vs. dCRC, as suggested in a previous study [21], we constructed a second model including age, sex, year of CRC diagnosis, and CRC stage at diagnosis. Survival probabilities and HRs were computed for one year and for one to five years after a CRC diagnosis. In addition, we investigated 90-day and five-year survival after a PCCRC/dCRC diagnosis.
Sensitivity analysis
Due to changing data availability over the study period, we identified T2D patients using both ICD-codes in the DNPR during 1977–2015 and prescription redemptions recorded in the DNHSPD during 2004–2015. We evaluated the impact of our identification method by conducting a sensitivity analysis restricted to patients who underwent colonoscopies during 2005–2015. In this analysis, we further evaluated the impact of our diabetes categorization (types 1 and 2) by extending the definition of type 1 diabetes mellitus to include patients with a DNPR diagnosis of diabetes before age 30, using insulin monotherapy, and with no history of oral glucose-lowering medications before the date of CRC diagnosis.
All data management and statistical analyses were conducted using Stata Version 15 (StataCorp, College Station, Texas, USA).
Results
Characteristics of PCCRCs vs. detected colorectal cancers
We identified 3088 T2D patients diagnosed with CRC during 1995–2015, who underwent a colonoscopy before their diagnosis. Characteristics of all T2D patients included in the study are shown in Table 1. A total of 250 (8.1%) patients were categorized as having PCCRC, and 2.838 (91.9%) patients were categorized as having dCRC. Although we observed a predominance of men in the study cohort (61%), PCCRC patients were more likely to be female than dCRC patients. Median age at CRC diagnosis was 74 years for patients with PCCRC and 73 years for patients with dCRC. The majority of PCCRCs (55%) were diagnosed during 2011-2015; however, the prevalence of PCCRC was higher than the prevalence of dCRCs at the beginning of the study period in the 1990s. Thus, PRs comparing PCCRC vs. dCRC declined over time, from 3.68 (95% CI 1.94–6.97) in 1995–2000 to 0.97 (95% CI 0.86–1.09) in 2011–2015 (Table 1).
Increased duration of T2D seemed to be associated with increasing prevalence of PCCRC. PCCRC patients were more likely than dCRC patients to have a high CCI score (the complete distribution of comorbidities included in the CCI is displayed in Supplementary Table 8), although atrial fibrillation/flutter and other cardiovascular diseases were highly prevalent in the entire T2D cohort. No difference was observed for diagnoses of obesity. In addition, we observed similar distributions of stage, histology, surgery, and MMR status among PCCRCs and dCRCs (Table 1). However, an increased prevalence of proximally located CRCs was observed for PCCRCs (PR: 1.43 [95% CI 1.27–1.62]). Similarly, PCCRC patients had an increased prevalence of histologically verified colorectal polyps and polypectomies recorded before the date of their CRC diagnosis (PR 2.19 [95% CI 1.96–2.45]) (Table 1).
Survival after post-colonoscopy vs. detected colorectal cancers
One-year survival after a CRC diagnosis was lower among T2D patients with PCCRC than among T2D patients with dCRC (Table 2). The crude HR for death at one year, comparing PCCRC with dCRC patients, was 1.85 (95% CI 1.47–2.31). When adjusting for age, sex, and year of CRC diagnosis, the association remained (HR 1.77 [95% CI 1.42–2.23]). The association also remained after inclusion of CRC stage in the regression model (HR 1.69 [95% CI 1.35–2.12]) (Table 2). Our additional analysis of 90-day survival after a CRC diagnosis showed the same pattern, with a crude HR of 1.95 (95% CI 1.43–2.65) and an age-, sex-, year-of-CRC-diagnosis-, and stage-adjusted HR of 1.71 (95% CI 1.25–2.33) (Supplementary Table 1).
One to five years after a CRC diagnosis, survival remained lower among T2D patients with PCCRC than among T2D patients with dCRC (Table 3). The estimated one- to five-year survival probability was 44% (95% CI 34–53) for patients with PCCRC and 54% (95% CI 52–57) for patients with dCRCs (Table 3). The corresponding adjusted HRs were 1.46 (95% CI 1.12–1.89) after adjusting for age, sex, and year of CRC, and 1.55 (95% CI 1.19–2.01) after adding stage to the model (Table 3). In our additional analysis of five-year survival, survival probabilities remained lower in the group of PCCRC patients. The corresponding five-year HR adjusted for age, sex, year of CRC diagnosis, and stage was 1.66 (96% CI 1.40–1.97) (Supplementary Table 2).
PCCRC patients had a poorer survival than dCRC patients across all strata of CCI scores (Tables 2 and 3).
Sensitivity analysis
The sensitivity analysis revealed no material differences from our main analysis. The results are shown in Supplementary Tables 3, 4, 5.
Discussion
In this registry-based nationwide cohort study of 3088 T2D patients diagnosed with either PCCRC or dCRC, we found that patients with PCCRC had an elevated prevalence of proximally located CRCs. PCCRCs and dCRCs did not differ in terms of stage, histology, and MMR status. The proportion of PCCRCs compared with dCRCs decreased over time. In all time-intervals analyzed, we found poorer survival in the PCCRC group compared with the dCRC group.
Previous research has suggested incidence of CRC and colorectal polyps to be linked with prevalent T2D as well as obesity [22,23,24]. Furthermore, Kort et al. studied the endoscopic phenotype and histopathology of colorectal polyps in patients with and without diabetes mellitus (including 3654 patients who underwent colonoscopy) [11]. They observed that diabetic patients more often had multiple and proximally located adenomas compared to non-diabetic patients. Our study found T2D-related PCCRCs to be more frequently diagnosed after resection of colorectal polyps than dCRCs. Considering the findings by Kort et al., our results study may reflect a predominance of T2D-related PCCRC caused by missed lesions at the index colonoscopy and particularly those missed in the proximal colon. The results of the present study thus expand those of Kort et al. and indicate the importance of specific surveillance and thorough preparation of patients with diabetes mellitus for colonoscopies.
We found that the proportion of PCCRCs decreased over time. This may indicate improved quality of colonoscopies [25]. Since CRC screening was introduced in Denmark in March 2014, there has been an increased focus on improving colonoscopy quality. In a large cohort study conducted using data from 2001 to 2015, Pedersen et al. found that the 3-year rate of PCCRC was generally higher in Denmark than in England, though declining over time [26]. Troelsen et al. reported the same decreasing trend [8].
Our survival analyses indicated that PCCRC was associated with poorer one-year and 1-to 5-year survival than dCRC. Despite conflicting results, the majority of previous studies found no difference in survival for patients with dCRC and PCCRC [2, 27,28,29,30,31,32]. These studies have led to the interpretation of PCCRC as CRC arising mainly from missed or insufficiently resected precursor lesions. In contrast, our findings indicate that T2D patients with PCCRC have a poorer prognosis than those with dCRC. This could indicate altered biology in the T2D patients related to hyperinsulinemia, hyperglycaemia, and changes in microbiota. Some studies suggested that long-term T2D might interfere with the molecular pathways of CRC, and that insulin sensitivity in T2D patients may lead to chronic compensatory hyperinsulinemia. Concurrent circulating levels of the insulin-like growth factor-1 receptor are associated with colorectal cancer development [33, 34]. Still, we found no difference between PCCRCs and dCRCs according to MMR status which has been associated with rapid tumor growth in previous research [35,36,37]. Our results concerning T2D-related CRC biology are thus contradictory and future studies on molecular characteristics are needed.
Study strengths include a population-based design and setting in Denmark, which has a well-organized health system generating continuously updated and high-quality data. Our study also has several limitations. First, we lacked data on quality of bowel preparation, completeness of the examination, endoscopist-specific adenoma detection rates, and information on surveillance regimens. This information is crucial for determining the clinical development of PCCRC, mainly because of possibly impaired bowel preparation prior to colonoscopy among T2D patients. Second, data availability differed during the study period, which may have affected our results. Data on prescriptions for diabetic medications were not available before 2004, when the DNHSPD was established [38].Thus up to 2004, our cohort only consisted of T2D patients with a diabetes diagnosis registered in the DNPR. For this reason, our study may have missed a proportion of T2D patients treated by general practitioners during 1995–2003. However, a sensitivity analysis restricted to colonoscopies performed during 2005–2012 indicated that this shortcoming had only a minor impact on our results.
In conclusion, our findings suggest overlooked colorectal lesions as a predominant explanation for T2D-related PCCRC, although altered tumor progression cannot be ruled out. This points to the need for increased awareness of colonoscopy quality and perhaps shorter intervals between colonoscopies among patients with T2D. Future research concerning T2D-related PCCRC should include information on quality of bowel preparation, completeness of colonoscopies, endoscopist-specific adenoma detection rates, and information on surveillance regimens.
Data availability
Data are available to legal residents of Denmark; however, only by application to the Danish Health Data Authority (https://sundhedsdatastyrelsen.dk/da/forskerservice/ansog-om-data) and the contributing hospitals. Hence, the data cannot be acquired directly by the authors.
Abbreviations
- CCI:
-
Charlson Comorbidity Index
- CRC:
-
Colorectal cancer
- CI:
-
Confidence interval
- T2D:
-
Type 2 diabetes mellitus
- dCRC:
-
Detected colorectal cancer
- HR:
-
Hazard ratio
- MMR:
-
Mismatch repair
- PCCRC:
-
Post-colonoscopy colorectal cancer
- PP:
-
Prevalence proportion
- PR:
-
Prevalence ratio
- SNOMED:
-
Systematized Nomenclature of Medicine
- DCR:
-
Danish Cancer Registry
- CRS:
-
Danish Civil Registration System
- ICD:
-
International Classification of Diseases
- ICD-8:
-
Eighth Revision
- ICD-10:
-
Tenth Revision
- DNHSPD:
-
Danish National Health Service Prescription Database
- DPR:
-
Danish National Pathology Registry
- DNPR:
-
Danish National Patient Registry
- WEO:
-
World Endoscopy Organization
References
Rutter MD, Beintaris I, Valori R et al (2018) World endoscopy organization consensus statements on post-colonoscopy and post-imaging colorectal cancer. Gastroenterology 155:909–25.e3
Erichsen R, Baron JA, Stoffel EM, Laurberg S, Sandler RS, Sorensen HT (2013) Characteristics and survival of interval and sporadic colorectal cancer patients: a nationwide population-based cohort study. Am J Gastroenterol 108:1332–1340
Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM (2012) Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer 118:3044–3052
Troelsen FS, Sørensen HT, Pedersen L, Erichsen R (2020) Risk of a post-colonoscopy colorectal cancer diagnosis in patients with inflammatory bowel disease: a Danish population-based cohort study. United Eur Gastroenterol J 8:22
Forsberg A, Widman L, Bottai M, Ekbom A, Hultcrantz R (2020) Postcolonoscopy colorectal cancer in Sweden from 2003 to 2012: survival, tumor characteristics, and risk factors. Clin Gastroenterol Hepatol 18:2724–33.e3
Laish I, Mizrahi J, Naftali T, Konikoff FM (2019) Diabetes mellitus and age are risk factors of interval colon cancer: a case-control study. Dig Dis 37:291–296
Suceveanu AI, Mazilu L, Nitipir C et al (2019) Diabetes mellitus raise the risk for interval colorectal cancer and advanced colorectal adenomas. Rev Chim 70:1808–1811
Troelsen FS, Sørensen HT, Pedersen L, Erichsen R (2021) Risk of a post-colonoscopy colorectal cancer in patients with type 2 diabetes: a Danish population-based cohort study. BMJ Open Gastroenterol 8:e000786
Horváth VJ, Putz Z, Izbéki F et al (2015) Diabetes-related dysfunction of the small intestine and the colon: focus on motility. Curr Diab Rep 15:94
Anderson R, Burr NE, Valori R (2020) Causes of post-colonoscopy colorectal cancers based on world endoscopy organization system of analysis. Gastroenterology 158:1287–99.e2
de Kort S, Bouwens MWE, Weijenberg MP et al (2017) Significantly higher rates of multiple and proximally located adenomas among patients with diabetes mellitus: a cross-sectional population-based study. United Eur Gastroenterol J 5:415–423
Ma Y, Yang W, Song M et al (2018) Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br J Cancer 119:1436–1442
Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8:915–928
World Health Organization. (2021) Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes.
Lin X, Xu Y, Pan X et al (2020) Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 10:14790
Schmidt M, Schmidt SAJ, Adelborg K et al (2019) The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 11:563–591
Schmidt M, Pedersen L, Sørensen HT (2014) The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 29:541–549
Pedersen CB (2011) The Danish civil registration system. Scand J Public Health 39:22–25
Thomsen RW, Sørensen HT (2015) Using registries to identify type 2 diabetes patients. Clin Epidemiol 7:1–3
Coutinho LM, Scazufca M, Menezes PR (2008) Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica 42:992–998
Stjarngrim J, Ekbom A, Hammar U, Hultcrantz R, Forsberg AM (2019) Rates and characteristics of postcolonoscopy colorectal cancer in the Swedish IBD population: what are the differences from a non-IBD population? Gut 68:1588–1596
Milek T, Forysinski K, Myrcha P, Ciostek P (2019) Diabetes association of polyps and colon cancer. Pol Przegl Chir 91:9–12
Soltani G, Poursheikhani A, Yassi M, Hayatbakhsh A, Kerachian M, Kerachian MA (2019) Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr Disord 19:113
Liang L, Liang Y, Li K et al (2022) A risk-prediction score for colorectal lesions on 12,628 participants at high risk of colorectal cancer. Gastroenterol Rep (Oxf). 10:goac002
Troelsen FS, Sørensen HT, Pedersen L et al (2023) Root-cause analysis of 762 Danish post-colonoscopy colorectal cancer patients. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2023.03.034
Pedersen L, Valori R, Bernstein I, Lindorff-Larsen K, Green C, Torp-Pedersen C (2019) Risk of post-colonoscopy colorectal cancer in Denmark: time trends and comparison with Sweden and the English National Health Service. Endoscopy 51:733–741
Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH (2006) Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 4:1259–1264
Gill MD, Bramble MG, Rees CJ, Lee TJW, Bradburn DM, Mills SJ (2012) Comparison of screen-detected and interval colorectal cancers in the bowel cancer screening programme. Br J Cancer 107:417–421
Samadder NJ, Curtin K, Tuohy TMF et al (2014) Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology 146:950–960
Dossa F, Sutradhar R, Saskin R et al (2020) Clinical and endoscopist factors associated with post-colonoscopy colorectal cancer in a population-based sample. Colorectal Dis. https://doi.org/10.1111/codi.15400
Macken E, Van Dongen S, De Brabander I, Francque S, Driessen A, Van Hal G (2019) Post-colonoscopy colorectal cancer in Belgium: characteristics and influencing factors. Endosc Int Open 7:E717–E727
Govindarajan A, Rabeneck L, Yun L, Tinmouth J, Paszat LF, Baxter NN (2016) Population-based assessment of the outcomes in patients with postcolonoscopy colorectal cancers. Gut 65:971–976
Murphy N, Carreras-Torres R, Song M et al (2020) Circulating levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 associate with risk of colorectal cancer based on serologic and Mendelian randomization analyses. Gastroenterology 158:1300–12.e20
Schoen RE, Weissfeld JL, Kuller LH et al (2005) Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology 129:464–475
Nakayama Y, Iijima T, Wakaume R et al (2019) Microsatellite instability is inversely associated with type 2 diabetes mellitus in colorectal cancer. PLoS ONE. https://doi.org/10.1371/journal.pone.0215513
Bogie RMM, Le Clercq CMC, Voorham QJM et al (2016) Association of chromosomal instability and microsatellite instability pathways with postcolonoscopy colorectal cancer in a retrospective cohort study. United European Gastroenterol J 4:A84–A85
Le Clercq C, Riedl R, Bouwens M et al (2013) Microsatellite instability, braf and Kras mutation in postcolonoscopy cancers: an explorative study. Gastroenterology 144:S283
Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M (2017) Data resource profile: the Danish National Prescription Registry. Int J Epidemiol 46:798
Funding
Open access funding provided by Aarhus University Hospital. The study was supported by grants from the Novo Nordisk Foundation (NNF19OC0058609) and the Danish Cancer Society (R247-A14719). The funding sources had no role in the design and conduct of the study, or the analysis and interpretation of the data.
Author information
Authors and Affiliations
Contributions
All authors contributed to the methodology of the study. HTS and RE acquired the data. FST and MLB directed the formal analyses, which were carried out by MLB. MLB wrote the initial draft. All authors contributed to the discussion and interpretation of the results. All authors reviewed, edited, and approved the final version for submission including the authorship list.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare.
Ethical approval
The study was reported to the Danish Data Protection Agency by Aarhus University (record No. 2016-051-000001/1671). According to Danish legislation, no ethical approval is needed for registry-based studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boysen, M.L., Troelsen, F.S., Sørensen, H.T. et al. Type 2 diabetes mellitus and post-colonoscopy colorectal cancer: clinical and molecular characteristics and survival. Cancer Causes Control 35, 1043–1052 (2024). https://doi.org/10.1007/s10552-024-01861-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-024-01861-9