Abstract
Purpose
Five-year relative survival for ovarian cancer remains below 50%. Strategies to improve outcomes are needed. Higher serum 25-hydroxyvitamin D [25(OH)D] concentrations [measure of vitamin D status] at and before diagnosis have been associated with longer survival in cancer patients; however, data for ovarian cancer are limited. We aimed to determine if 25(OH)D concentrations during and after primary treatment were associated with ovarian cancer-specific survival.
Methods
We used data from a nationwide prospective cohort study of women with ovarian cancer. Among 886 participants treated with chemotherapy, 700 (79%) had a blood sample collected during (n = 591) and/or after (n = 458) primary treatment. These were tested for 25(OH)D. Clinical and survival data were abstracted from medical records. We used multivariable Cox proportional hazards regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) for associations between 25(OH)D and ovarian cancer-specific survival.
Results
Mean 25(OH)D concentrations were lower during than after primary treatment (82 and 91 nmol/L, respectively); only 14% and 8% had concentrations below 50 nmol/L during and after primary treatment, respectively. There was no association between 25(OH)D and ovarian cancer-specific survival during five years of follow-up [HR 1.10 (95% CI: 0.76, 1.61) and 0.95 (0.54, 1.68) for the highest vs. lowest quintile during and after treatment, respectively].
Conclusions
We did not observe any association between serum 25(OH)D concentration and ovarian cancer-specific survival. Our results suggest that, in the absence of vitamin D deficiency, vitamin D supplementation to improve ovarian cancer survival is not warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the eight most common incident cancer and seventh most common cause of cancer death in the female population worldwide [1]. More than two-thirds of those affected are diagnosed with advanced disease [2] and while the majority of patients respond to primary treatment, most will relapse. Despite a modest improvement over the last 25 years, 5-year relative survival remains below 50% in Australia and other high income countries [3,4,5].
When bound to the active form of vitamin D, the vitamin D receptor (VDR) plays a role in regulating the expression of more than 900 genes, which are involved in calcium homeostasis, control of cell cycling and growth, cellular differentiation, and immune response [6]. High expression of VDR has been seen in ovarian cancer cells [7, 8], and the active form of vitamin D has been shown to inhibit cell proliferation and induce apoptosis in ovarian cancer cells in vitro [7, 9], although at much higher concentrations than occur naturally. Furthermore, platinum and taxane agents showed greater inhibition of ovarian cancer cell proliferation with the addition of high concentrations of active vitamin D [7]. This suggests vitamin D may improve the response to chemotherapy in ovarian cancer.
Randomised controlled trials (RCTs) of vitamin D supplementation and cancer mortality have had mixed results, with two recent meta-analyses finding no significant association with cancer mortality overall [10, 11], although a benefit was seen for lung cancer mortality [10] and in the subset of trials that used daily dosing [11]. Neither meta-analysis reported ovarian cancer mortality specifically, nor have any trials examined cancer survival. A meta-analysis of prospective cohort studies found higher concentrations of 25(OH)D (presumably pre-diagnosis) were associated with reduced cancer mortality [12]. This meta-analysis did not look at the association by cancer site, but did find a stronger association among the female population. A more recent observational study found higher pre-diagnosis 25(OH)D concentrations were associated with improved overall cancer survival; however, in site-specific analysis a significant benefit was only seen for lung cancer with no association in the small (n = 74) subgroup with ovarian cancer [13]. In contrast, 25(OH)D concentrations less than 50 nmol/L at diagnosis, but not after treatment, were associated with poorer survival in one ovarian cancer study (n = 670 at diagnosis and n = 336 after treatment) [14].
If a survival benefit with higher 25(OH)D concentration is confirmed, vitamin D supplementation could be recommended for ovarian cancer patients. The period during primary treatment is of particular relevance as this is when 25(OH)D concentrations are likely to be lowest due to increased time indoors recovering from surgery and receiving chemotherapy, and in vitro studies have suggested 25(OH)D may have an additive benefit to chemotherapy [7]. We, therefore, aimed to determine if serum 25(OH)D concentrations: (i) during; and (ii) after primary treatment are associated with survival in women with ovarian cancer. We hypothesised that low 25(OH)D concentrations would be associated with poorer survival.
Methods
Participants
The Ovarian Cancer, Prognosis and Lifestyle (OPAL) study is a national prospective cohort study of 958 Australian women aged 18–79 years with a diagnosis of primary invasive epithelial ovarian, peritoneal or fallopian tube cancer between January 2012 and April 2015. Participants were identified through major treatment centres and approached as soon as possible after diagnosis. They were asked to complete questionnaires at recruitment (T0), then at three-monthly intervals for the first year after diagnosis (T3, T6, T9, T12), then annually (T24, T36, T48). From these we obtained sociodemographic (T0), medical and lifestyle data. Clinical data, treatment information, disease recurrence and vital status were abstracted annually from women’s medical records. Blood samples were collected at recruitment (median 2 and 3 months after the start of primary treatment for those undergoing adjuvant and neoadjuvant treatment, respectively), and again at 12 months after diagnosis if women were recurrence-free (median 8 and 6 months after the end of primary treatment for adjuvant and neoadjuvant treatment, respectively). Approval was obtained from the Human Research Ethics Committees of QIMR Berghofer Medical Research Institute and all participating centres. Participants provided informed consent.
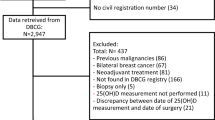
Figure 1 shows the flow of participants through to analysis. Only participants with invasive disease who underwent chemotherapy as part of primary treatment, had recorded dates for initiation and completion of primary treatment, and at least 3 months of follow-up data were eligible for inclusion (n = 886, Fig. 1). We considered blood samples to be ‘during’ primary treatment if they were collected any time between the start of any treatment to within 30 days of finishing primary treatment. Samples were considered ‘after’ primary treatment if collected 31 days to 18 months after finishing primary treatment (and before recurrence). We excluded samples taken after recurrence or progression from the ‘after’ primary treatment analysis to avoid including those collected while the participant was on active treatment. The mean time from the start of treatment to collection of bloods was 2.5 months (SD 1.5) and 12.6 months (SD 2.3) for samples classified as ‘during’ and ‘after’ primary treatment, respectively. Participants who did not provide a blood sample (n = 131), and those that did not have a sample suitable for testing collected during or after primary treatment as defined above (n = 55) were excluded. Of the 886 eligible participants, 700 (79%) had one (351) or two (349) eligible blood samples, with 591 samples collected during primary treatment; and 458 samples collected after primary treatment and before recurrence.
25(OH)D concentrations
Samples were tested by a laboratory involved in the international Vitamin D Standardization Program using liquid chromatography tandem mass spectroscopy [15]. This measures 25(OH)D2 (ergocalciferol) and 25(OH)D3 (cholecalciferol). Total 25(OH)D (ergocalciferol + cholecalciferol) was used for this analysis. As there is notable seasonal variation in 25(OH)D concentrations we used a previously described technique to deseasonalize measures [16]; we did this separately for the samples collected during and after treatment as mean 25(OH)D differed between the two groups. 25(OH)D concentrations of 50 nmol/L or more are considered sufficient according to current guidelines; however, this cut-point was derived using older immunoassays and may not apply to newer assays such as that used here, which tends to give higher values. We thus categorised the deseasonalized 25(OH)D concentrations into quintiles for the primary analysis. For comparison with other studies we also created the following categories: less than 50 nmol/L; 50–74.9 nmol/L; 75–99.9 nmol/L; and 100 nmol/L or more.
Survival
Survival was measured from the start of treatment to the latest date a woman was last known to be alive or truncated at five years after the start of primary treatment. Only 12 individuals were lost to follow-up during this five-year interval [their mean follow-up was 45.4 months (range 24.0 – 59.8)]. We assessed ovarian cancer-specific survival (OCS) to exclude any effect vitamin D may have on all-cause mortality. Those who died from other causes (n = 6, 1%) were censored at the time of death.
Statistical analysis
We used Cox proportional hazards regression models to calculate hazard ratios (HR) and 95% confidence intervals (95% CI) for the association between 25(OH)D (i) during and (ii) after primary treatment, and ovarian cancer-specific survival. Survival time was left-truncated to the date of blood collection to avoid immortal time bias. Covariate inclusion was informed by a directed acyclic graph (DAG) and linear regression to identify factors associated with deseasonalized 25(OH)D in the included population (Supplementary Table 1). As not all lifestyle information was collected at every questionnaire time-point, and some participants were recruited several months after diagnosis and missed some questionnaires, up to 25% of participants were missing measures of current physical activity, time spent outdoors or vitamin supplementation around the time of blood collection. As these factors did not appreciably alter the estimates of association between 25(OH)D and OCS in complete case analyses, they were not included in the final models. All models were adjusted for age at diagnosis and conditioned on FIGO stage of disease at diagnosis to allow the baseline hazard to vary by stage. Fully-adjusted models were additionally adjusted for body mass index (BMI) (< 25 kg/m2, 25–29.9 kg/m2, ≥ 30 kg/m2) 5 years prior to diagnosis, smoking status at the time of recruitment (ever versus never smokers), and Charlson comorbidity index [17] based on self-reported medical conditions prior to diagnosis (0, 1, ≥ 2). Proportional hazards assumptions testing indicated the association between smoking status and OCS varied slightly over time; however, inclusion of an interaction term for smoking status × time did not alter the magnitude of the associations between 25(OH)D and survival, and this was therefore omitted from the final models.
We also analysed the association between serum 25(OH)D concentration during treatment and ovarian cancer survival including all eligible participants (n = 886) using multiple imputation to impute missing 25(OH)D quintile (33%) and covariate data (7%). Those missing a blood sample on treatment were slightly more likely to be of Asian ethnicity (9 vs. 4%), have 2 or more significant comorbidities (14 vs. 8%) and a low physical activity index prior to diagnosis (54 vs. 46%) than those with blood samples, but were similar in other characteristics. Under the assumption of missing at random, we used the multivariate imputation by chained equations (MICE) method [18], and included the previously identified covariates and the following auxiliary variables in the imputation model: ethnicity, education, FIGO stage and histotype at diagnosis, season of blood collection, deseasonalized 25(OH)D quintile, physical activity, time spent outdoors, any supplementation and vitamin D supplementation at or around the time of blood collection, and the cumulative baseline cause-specific hazard and binary indicator variable for the outcome [19, 20]. We set the time of blood collection for imputed values to the median time among those with samples (2 and 3 months after the start of primary treatment for those receiving adjuvant and neoadjuvant treatment, respectively). We imputed 20 datasets and combined the resulting estimates and standard errors using Rubin’s rules [21].
Ethnicity may also affect 25(OH)D and survival (via histotype), but as 89% of the included population were of white ethnicity this was not included in models. We conducted a subgroup analysis to determine if the association was the same when only those of white ethnicity were included. Further analyses were restricted to those: (i) with FIGO stage III and IV disease; (ii) who had cytoreductive surgery as part of treatment; and (iii) taking less than 500 IU/day of supplemental vitamin D daily around the time of blood collection.
Imputation and analysis of imputed data was completed using R statistical software (version 4.1.3, R Development Core Team, 2022). Other analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
The mean age of included participants (n = 700) at diagnosis was 60 years, 75% had advanced stage disease, and 77% had high-grade serous carcinoma (Supplementary Table 2). 22% were taking daily vitamin D supplements of at least 500IU (12.5 micrograms cholecalciferol, indicative of regular supplementation) prior to diagnosis (Supplementary Table 2). Eligible participants who were excluded were slightly older (mean age 62 vs. 60 years), and more likely to be of Asian ethnicity (10 vs. 4%), have multiple comorbidities (16 vs. 8%), be current smokers (10 vs. 4%), and to have received chemotherapy only (9 vs. 3%), than those included (Supplementary Table 2). Otherwise the excluded women were very similar. The demographic and clinical characteristics of participants included in the analyses of 25(OH)D during and after primary treatment were very similar, except slightly fewer had primary surgery and adjuvant chemotherapy (65 vs. 72%) in the during-treatment analysis (Table 1).
Mean deseasonalized 25(OH)D concentrations were lower during primary treatment [82 nmol/L (SD 32 nmol/L)], than after treatment [91 nmol/L (SD 32 nmol/L)]. Only 14% and 8% of samples had concentrations below 50 nmol/L during and after primary treatment, respectively. Five-year OCS was 55% (260 ovarian cancer deaths) and 74% (118 ovarian cancer deaths) for participants included in during-treatment and after-treatment analyses, respectively (Table 2). This difference was expected, as women included in the after-treatment analysis had survived an average of 12.6 months without recurrence when their post-treatment bloods were collected. Mean follow-up time contributing to analyses for those alive at last contact was 59.6 months and 59.7 months for during-treatment and after-treatment analyses, respectively.
Table 2 shows the results of the main analysis. While the HR was below one among those in the fourth quintile of 25(OH)D during treatment (0.72; 95% CI: 0.48, 1.07) compared to the lowest quintile, the HRs for the other quintiles were all above one and there was no linear trend with increasing 25(OH)D. Analysis using imputed 25(OH)D quintiles for those missing blood samples during primary treatment did not change the results (Supplementary Table 3).
There was no association between 25(OH)D and OCS in analysis of 25(OH)D concentration measured after treatment (HR 0.95; 95% CI: 0.54, 1.68; highest compared to lowest quintile) (Table 2). There was no increase in ovarian cancer mortality in those with concentrations below 50 nmol/L in during-treatment or after-treatment analyses (Supplementary Table 4).
When we excluded those taking 500 IU or more of vitamin D supplementation, those with 25(OH)D concentrations in the fourth quintile during primary treatment (89.2–105.8 nmol/L) appeared to have better survival (HR 0.60; 95% CI: 0.34–1.07) compared to those in the lowest quintile; however, this was not seen in the highest quintile and there was no trend across quintiles (Table 3). After primary treatment, the HR for quintiles two to five compared to the lowest quintile decreased with increasing 25(OH)D; however, they were all over 1.0, except quintile five (HR: 0.85; 95% CI: 0.40, 1.80) (Table 3). The results were essentially unchanged when we restricted the population to white ethnicity, FIGO stage III and IV disease, and those that received cytoreductive surgery (Table 3).
Discussion
We did not find evidence of an association between 25(OH)D concentrations in blood samples collected during or after primary treatment and OCS in this Australian population. However, vitamin D deficiency [currently defined as 25(OH)D < 50 nmol/L] was comparatively rare; thus we had limited power to detect an association between vitamin D deficiency and survival.
To the best of our knowledge, no other study has examined 25(OH)D concentrations during treatment and ovarian cancer survival, and only one other has used measures from samples collected after treatment. Our after-treatment results are consistent with a previous Australian study that did not find a significant association between 25(OH)D concentrations after treatment and survival [14]. While not directly comparable, our during-treatment results are consistent with a previous small study (n = 74) that found no association between pre-diagnosis serum 25(OH)D concentrations and ovarian cancer survival (HR 0.93; 95% CI: 0.41, 2.09; highest vs. lowest tertile) [13]. However, they are not consistent with the earlier Australian study that found lower 25(OH)D concentrations at diagnosis were associated with poorer survival in ovarian cancer (< 25 nmol/L HR 1.44; 95% CI: 1.07, 1.95 and 25–49.9 nmol/L HR 1.30; 95% CI: 1.01, 1.66; compared to 50.0–74.9 nmol/L) [14]. One other small study (n = 72) also found ovarian cancer patients with low (< 25 nmol/L) 25(OH)D before primary treatment had poorer survival compared to those with concentrations of 25 nmol/L or more; however that analysis was not adjusted for confounders [22]. The differing timing of sampling of these studies may have contributed to the differences in results.
In both previous studies that found lower 25(OH)D concentrations were associated with poorer survival in ovarian cancer [14, 22], vitamin D deficiency was much more common. It is plausible that this difference is at least partly due to the different methods used to measure 25(OH)D. However, the use of vitamin D supplementation (≥ 500 IU daily) has increased in the ten years between the first Australian study (< 1%; personal communication) and this study (22%), so it is also likely that there are true differences in 25(OH)D concentrations between the two.
It is possible that any association between 25(OH)D and cancer survival may be affected by genetic factors. If this were the case, then genetic differences in study populations could contribute to inter-study differences. A recent study observed improved overall cancer and lung cancer survival in those with higher pre-diagnosis 25(OH)D concentrations, but only among those with particular vitamin D binding protein (VDBP) isoforms [13]. Polymorphisms in the VDR may also influence associations between 25(OH)D and cancer survival. An association between VDR polymorphisms and survival in ovarian cancer patients has previously been observed in a small study (n = 101) [23]. Further exploration of how VDBP and VDR polymorphisms may influence the relationship between 25(OH)D concentrations and survival is warranted.
A strength of our study is the use of a gold-standard measure of 25(OH)D, which is likely to be more accurate than measures used in older studies [15]. As samples were collected at different times of the year we also used a technique to deseasonalize 25(OH)D concentrations to reduce the risk of misclassifying overall vitamin D status, particularly if a sample had been collected at the end of winter or summer. However, our study was limited by low power to detect an association between vitamin D deficiency and survival as only a small proportion of the study population had 25(OH)D concentrations less than 50 nmol/L.
Conclusion
Our results do not support an association between 25(OH)D concentrations and OCS at the population level and do not replicate a trend towards better survival in those with higher 25(OH)D concentrations seen in a previous cohort. Our results do not support universal vitamin D supplementation of ovarian cancer patients in a largely vitamin D sufficient population such as Australia.
Data availability
The datasets generated during and/or analysed during the current study are not available as open access as participants did not consent to this.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Anuradha S, Webb PM, Blomfield P, Brand AH, Friedlander M, Leung Y et al (2014) Survival of australian women with invasive epithelial ovarian cancer: a population-based study. Med J Aust 201(5):283–288. https://doi.org/10.5694/mja14.00132
Australian Institute of Health and Welfare (2022) Cancer data in Australia. AIHW, Canberra. https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia Accessed 1 Dec 2022
Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust T et al (2019) Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 20(11):1493–1505. https://doi.org/10.1016/s1470-2045(19)30456-5
American Cancer Society (2022) Survival Rates for Ovarian Cancer: American Cancer Society https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html. Accessed 1 Dec 2022
von Essen MR, Geisler C (2018) VDR, the vitamin D receptor. In: Choi S (ed) Encyclopedia of signaling molecules. Springer, Cham, pp 5907–5914
Kuittinen T, Rovio P, Luukkaala T, Laurila M, Grénman S, Kallioniemi A et al (2020) Paclitaxel, carboplatin and 1,25-D3 inhibit proliferation of ovarian cancer cells in Vitro. Anticancer Res 40(6):3129–3138. https://doi.org/10.21873/anticanres.14294
Villena-Heinsen C, Meyberg R, Axt-Fliedner R, Reitnauer K, Reichrath J, Friedrich M (2002) Immunohistochemical analysis of 1,25-dihydroxyvitamin-D3-receptors, estrogen and progesterone receptors and Ki-67 in ovarian carcinoma. Anticancer Res 22(4):2261–2267
Thill M, Woeste A, Reichert K, Fischer D, Rody A, Friedrich M et al (2015) Vitamin D inhibits ovarian cancer cell line proliferation in combination with celecoxib and suppresses cyclooxygenase-2 expression. Anticancer Res 35(2):1197–1203
Zhang R, Zhang Y, Liu Z, Pei Y, Xu P, Chong W et al (2022) Association between vitamin D supplementation and cancer mortality: a systematic review and meta-analysis. Cancers. https://doi.org/10.3390/cancers14153717
Keum N, Chen QY, Lee DH, Manson JE, Giovannucci E (2022) Vitamin D supplementation and total cancer incidence and mortality by daily vs. infrequent large-bolus dosing strategies: a meta-analysis of randomised controlled trials. Br J Cancer 127(5):872–878. https://doi.org/10.1038/s41416-022-01850-2
Han J, Guo X, Yu X, Liu S, Cui X, Zhang B et al (2019) 25-Hydroxyvitamin D and total cancer incidence and mortality: a meta-analysis of prospective cohort studies. Nutrients. https://doi.org/10.3390/nu11102295
Weinstein SJ, Mondul AM, Layne TM, Yu K, Huang J, Stolzenberg-Solomon RZ et al (2022) Prediagnostic serum vitamin D, vitamin D binding protein isoforms, and cancer survival. JNCI Cancer Spectr. https://doi.org/10.1093/jncics/pkac019
Webb PM, de Fazio A, Protani MM, Ibiebele TI, Nagle CM, Brand AH et al (2015) Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. Am J Clin Nutr 102(1):109–114. https://doi.org/10.3945/ajcn.114.102681
Clarke MW, Tuckey RC, Gorman S, Holt B, Hart PH (2013) Optimized 25-hydroxyvitamin D analysis using liquid–liquid extraction with 2D separation with LC/MS/MS detection, provides superior precision compared to conventional assays. Metabolomics 9(5):1031–1040. https://doi.org/10.1007/s11306-013-0518-9
Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N et al (2013) Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the multi-ethnic study of atherosclerosis. Am J Clin Nutr 97(6):1243–1251. https://doi.org/10.3945/ajcn.112.054502
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251. https://doi.org/10.1016/0895-4356(94)90129-5
Van Buuren S, Groothuis-Oudshoorn K (2011) Mice: multivariate imputation by chained equations in R. J Stat Softw 45:1–67. https://doi.org/10.18637/jss.v045.i03
White IR, Royston P (2009) Imputing missing covariate values for the Cox model. Stat Med 28(15):1982–1998. https://doi.org/10.1002/sim.3618
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30(4):377–399. https://doi.org/10.1002/sim.4067
Rubin DB (2004) Multiple imputation for nonresponse in surveys. Wiley, Hoboken
Walentowicz-Sadlecka M, Grabiec M, Sadlecki P, Gotowska M, Walentowicz P, Krintus M et al (2012) 25(OH)D3 in patients with ovarian cancer and its correlation with survival. Clin Biochem 45(18):1568–1572. https://doi.org/10.1016/j.clinbiochem.2012.07.110
Tamez S, Norizoe C, Ochiai K, Takahashi D, Shimojima A, Tsutsumi Y et al (2009) Vitamin D receptor polymorphisms and prognosis of patients with epithelial ovarian cancer. Br J Cancer 101(12):1957–1960. https://doi.org/10.1038/sj.bjc.6605414
Acknowledgments
We acknowledge the OPAL study team and all the clinicians and participating institutions (see opalstudy.qimrberghofer.edu.au). We thank consumer representatives Merran Williams, Karen Livingstone and Hélène O’Neill for their conceptual input and most of all the OPAL participants that made this study possible.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The OPAL study was funded by the National Health and Medical Research Council (NHMRC) of Australia (GNT1025142, GNT1073898) and Brisbane Women’s Club. The Donald and Joan Wilson Foundation provided funding for 25(OH)D testing. PM Webb and R Na were supported by an NHMRC Investigator Grant (GNT1043134). TL Ross was supported by a NHMRC Postgraduate Scholarship (GNT2014122).
Author information
Authors and Affiliations
Consortia
Contributions
TLR, PMW, REN, and RN contributed to the study conception and design. TLR conducted the analysis and wrote the manuscript. All authors contributed to data interpretation, and critically reviewed previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
PM Webb has received grant funding from AstraZeneca for an unrelated study of ovarian cancer and a speakers fee from AstraZeneca (Dec 2021).
Ethical approval
Approval was obtained from the Human Research Ethics Committees of QIMR Berghofer Medical Research Institute and all participating centres.
Informed consent
was obtained from all participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ross, T.L., Neale, R.E., Na, R. et al. Vitamin D status during and after treatment and ovarian cancer survival. Cancer Causes Control 35, 1–8 (2024). https://doi.org/10.1007/s10552-023-01757-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-023-01757-0