Abstract
Purpose
To investigate differences in prescription rates of commonly used drugs among prostate cancer patients and cancer-free comparisons and between patients diagnosed with localized and non-localized disease.
Methods
We conducted a register-based study including all men aged 50–85 years diagnosed with prostate cancer in Denmark from 1998 to 2015 and an age-matched cancer-free comparison cohort. We calculated the number of new and total prescriptions from three years before to three years after the date of diagnosis of the case for selected drug classes divided by the number of person-months and stratified by stage at diagnosis.
Results
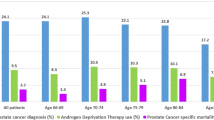
We included 54,286 prostate cancer patients and 249,645 matched comparisons. 30,712 patients were diagnosed with localized disease and 12,884 with non-localized disease. The rates of new prescriptions increased considerably among patients within the year before the diagnosis. Hereafter the rates varied between drug classes. For most drug classes, total prescription rates for patients and comparisons increased similarly in the study period. Total prescription rates varied between men with localized and non-localized disease for all drug classes apart from statins.
Conclusion
Our findings indicate that a large proportion of prostate cancer cases are likely diagnosed during medical work-up for other reasons than prostate cancer. Increased rates occur within the last year before diagnosis and future studies on the interaction between drug use and prostate cancer should at least include a one year pre-diagnostic lag-time. Post-diagnostic prescription rates demonstrated an increased use of drugs most likely associated with the consequences of the disease.






Similar content being viewed by others
Data availability
Access to data can be obtained by request to the corresponding authors and the Danish data protection authorities.
Code availability
The code can be obtained by request to the corresponding author.
References
Daskivich TJ, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Stanford JL, Stroup AM, Litwin MS, Penson DF (2013) Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med 158:709–717. https://doi.org/10.7326/0003-4819-158-10-201305210-00005
Loeppenthin K, Dalton SO, Johansen C, Andersen E, Christensen MB, Pappot H, Petersen LN, Thisted LB, Frølich A, Mortensen CE, Lassen U, Ørsted J, Bidstrup PE (2020) Total burden of disease in cancer patients at diagnosis—a Danish nationwide study of multimorbidity and redeemed medication. Br J Cancer 123:1033–1040. https://doi.org/10.1038/s41416-020-0950-3
Gedeborg R, Garmo H, Robinson D, Stattin P (2020) Prescription-based prediction of baseline mortality risk among older men. PLoS ONE 15:e0241439. https://doi.org/10.1371/journal.pone.0241439
Pottegård A, Hallas J (2017) New use of prescription drugs prior to a cancer diagnosis. Pharmacoepidemiol Drug Saf 26:223–227. https://doi.org/10.1002/pds.4145
Pedersen CB (2011) The Danish civil registration system. Scand J Public Heal 39:22–25. https://doi.org/10.1177/1403494810387965
Gjerstorff ML (2011) The Danish cancer registry. Scand J Public Health 39:42–45. https://doi.org/10.1177/1403494810393562
Kildemoes HW, Sørensen HT, Hallas J (2011) The Danish national prescription registry. Scand J Public Health 39:38–41. https://doi.org/10.1177/1403494810394717
Friberg AS, Dalton SO, Larsen SB, Andersen EW, Krøyer A, Helgstrand JT, Røder MA, Johansen C, Brasso K (2019) Risk of depression after radical prostatectomy—a nationwide registry-based study. Eur Urol Oncol 4:6–13. https://doi.org/10.1016/j.euo.2019.06.020
Yu R, Li H (2021) Longitudinal assessment of prevalence and risk factors of anxiety and depression among prostate cancer survivors post-resection. Psychiatr Q 92:1–15. https://doi.org/10.1007/s11126-020-09869-5
Yiannopoulou KG, Anastasiou AI, Kontoangelos K, Papageorgiou C, Anastasiou IP (2020) Cognitive and psychological impacts of different treatment options for prostate cancer: a critical analysis. Curr Urol 14:169–177. https://doi.org/10.1159/000499242
Friberg AS, Brasso K, Larsen SB, Andersen EW, Krøyer A, Helgstrand JT, Røder MA, Klemann N, Kessing LV, Johansen C, Dalton SO (2021) Risk of depression after diagnostic prostate cancer workup—a nationwide, registry-based study. Psychooncology. https://doi.org/10.1002/pon.5766
Røder MA, Brasso K, Berg KD, Thomsen FB, Gruschy L, Rusch E, Iversen P (2013) Patients undergoing radical prostatectomy have a better survival than the background population. Dan Med J 60:4612
Lerhmann-Lerche CS, Larsen SB, Andersen I, Thygesen LC, Kaae Andersen K, Duun-Henriksen AK, Johansen C, Røder MA, Brasso K, Dalton SO (2019) Educational level and first-time PSA testing in general practice. Scand J Urol 53:275–281. https://doi.org/10.1080/21681805.2019.1681503
Karlsen RV, Larsen SB, Christensen J, Brasso K, Friis S, Tjonneland A, Dalton SO (2013) PSA testing without clinical indication for prostate cancer in relation to socio-demographic and clinical characteristics in the Danish diet, cancer and health study. Acta Oncol 52:1609–1614. https://doi.org/10.3109/0284186X.2013.831474
Larsen SB, Brasso K, Christensen J, Johansen C, Tjønneland A, Friis S, Iversen P, Dalton SO (2017) Socioeconomic position and mortality among patients with prostate cancer: influence of mediating factors. Acta Oncol 56:563–568. https://doi.org/10.1080/0284186X.2016.1260771
Schmidt M, Pedersen L, Sorensen HT (2014) The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol 29:541–549. https://doi.org/10.1007/s10654-014-9930-3
Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, Sørensen HT, Hallas J, Schmidt M (2017) Data resource profile: the Danish national prescription registry. Int J Epidemiol 46:798. https://doi.org/10.1093/ije/dyw213
Funding
The present study was funded by research grants from Kirsten and Freddy Johansens Fond and from the Peer Kølendorf Family Foundation. Anne Katrine Duun-Henriksen was funded by a research Grant from the Danish Cancer Society Scientific Committee (R204-A12.653).
Author information
Authors and Affiliations
Contributions
Study idea and design: SBL, CD, KB, AP, MAR, SF, AKDH. Statistical analyses: CD, AKDH. Writing manuscript: SBL, CS, KB, AKDH. Interpretation and critical review of the manuscript: SBL, CD, CS, SF, AP, MAR, KB, AKDH. All authors had read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not needed for register-based studies.
Consent to participate
NA
Consent for publication
All authors have approved the final version of the manuscript before submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Larsen, S.B., Dehlendorff, C., Skriver, C. et al. Prescription rates for commonly used drugs before and after a prostate cancer diagnosis. Cancer Causes Control 33, 417–428 (2022). https://doi.org/10.1007/s10552-021-01537-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-021-01537-8