Abstract
Purpose
Ki-67 is recommended by international/national guidelines for risk stratification in early breast cancer (EBC), particularly for defining “intermediate risk,” despite inter-laboratory/inter-observer variability and cutoff uncertainty. We investigated Ki-67 (> 10%– < 40%, determined locally) as a prognostic marker for intermediate/high risk in EBC, pN0-1 patients.
Methods
This prospective, non-interventional, real-world study included females ≥ 18 years, with pN0/pN1mi/pN1, HR+ , HER2-negative EBC, and locally determined Ki-67 ranging 10%–40%. The primary outcome was changes in treatment recommendations after disclosing the Oncotype DX Breast Recurrence Score®(RS) assay result.
Results
The analysis included 567 patients (median age, 57 [range, 29–83] years; 70%/1%/29%/ with pN0/pN1mi/pN1 disease; 81% and 19% with RS results 0–25 and 26–100, respectively). The correlations between local and central Ki-67, local Ki-67, and the RS, and central Ki-67 and the RS results were weak (r = 0.35, r = 0.3, and r = 0.46, respectively), and discrepancies were noted in both directions (e.g., local Ki-67 was lower or higher than central Ki-67). After disclosing the RS, treatment recommendations changed for 190 patients (34%). Changes were observed in pN0 and pN1mi/pN1 patients and in patients with centrally determined Ki-67 ≤ 10% and > 10%. Treatment changes were aligned with RS results (adding chemotherapy for patients with higher RS results, omitting it for lower RS results), and their net result was 8% reduction in adjuvant chemotherapy use (from 32% pre-RS results to 24% post-RS results).
Conclusion
The Oncotype DX® assay is a tool for individualizing treatments that adds to classic treatment decision factors. The RS result and Ki-67 are not interchangeable, and Ki-67, as well as nodal status, should not be used as gatekeepers for testing eligibility, to avoid under and overtreatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sole use of classic prognostic factors to guide adjuvant treatment decisions and predict the risk of recurrence in breast cancer has been a practice for over 30 years [1,2,3,4,5]. The initial prognostic factors used included tumor size, nuclear and histologic grade, as well as biomarkers such as estrogen receptor (ER) and progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) amplification, and cathepsin D levels [1,2,3,4,5].
In recent years, growing evidence demonstrated that the biomarker pathways are interconnected/codependent. Therefore, assays evaluating multiple biomarkers simultaneously and weighting them appropriately, could be used to more accurately estimate the recurrence risk of an individual patient.
Among others, the 21-gene Breast Recurrence Score® assay is a validated multigene assay that measures the expression of 16 cancer genes and 5 reference genes and takes into account their interconnection/codependency in the calculation of the recurrence score (RS) result from normalized gene expression levels. The RS result ranges from 0 to 100, with higher scores associated with an increased recurrence risk. Patients with high RS results were also shown to gain a substantial benefit from adjuvant chemotherapy (CT) followed by endocrine therapy (ET) [6,7,8,9,10,11,12]. The 21-gene assay has been used in clinical practice to adapt adjuvant treatment recommendations in patients with early-stage hormone receptor (HR) + breast cancer since 2004 [13,14,15].
The use of the proliferation marker Ki-67 (as determined by immunohistochemistry) as a potential guidance in adjuvant treatment decisions has been extensively investigated since it was first introduced in the 1980s, and this evolution is reflected in international guidelines. However, in spite of all attempts at standardizing the assessment of Ki-67, its use remains limited due to inter-laboratory/inter-observer variability and cutoff uncertainty [16,17,18]. The current US National Comprehensive Cancer Network®(NCCN) guidelines for breast cancer accordingly do not recommend routine assessment of Ki-67 [19]. The International Ki-67 in Breast Cancer Working Group (IKWG) published updated recommendations in 2021. These recommendations acknowledged the questionable analytical validity of Ki-67, recommended approaches to mitigate this limitation (e.g., preanalytical handling considerations, standardized visual scoring, participation in programs for quality assurance/control), and stated that in T1-2, N0-1 ER + HER2-negative patients, Ki-67 ≤ 5% or ≥ 30%, can be used to estimate prognosis [20]. The first prospective study comparing the performance of the Oncotype DX assay results and centrally determined immunohistochemistry markers including Ki-67 was the WSG Plan-B Trial in which disease-free survival (DFS) was investigated by RS result in correlation with Ki-67. The results suggested that RS results had the highest prognostic value among patients with intermediate Ki-67 levels (> 10% to < 40%) [21].
Here, we report on the Rhein-Main-Registry (REMAR) prospective, real-world German study focusing on patients with Ki-67 in the intermediate range (10–40%, as determined locally) and evaluated the reliability of this Ki-67 range as a marker to identify intermediate- or high-risk pN0-1 breast cancer patients who are likely to benefit from the prognostic/predictive information gained by the RS result. Participating clinicians were blinded to the RS results and assigned the patients to ET alone or ET plus CT (CET) based on clinicopathologic factors, including the local Ki-67 value, in their institutional multidisciplinary tumor boards, and re-evaluated the treatment decisions post-surgery when the RS results became available within a second round within the same institutional multidisciplinary tumor board.
Methods
Study design
The REMAR study was a prospective, non-interventional real-world cohort study conducted in the Rhein-Main region of Germany, which has a population of approximately 5.8 million people. The study included 15 certified breast cancer centers in this region.
The primary outcome of the study was the change in adjuvant treatment recommendations, as determined by the multidisciplinary tumor boards of the participating institutions, after the RS result became available. Other outcomes included the correlation between local and central Ki-67 testing and between the RS result and Ki-67 levels (as determined locally and centrally).
The study protocol was approved by the Ethics Committee at the Board of Physicians in Hesse, Germany (study number FF48/2015), and informed consent was obtained from all participating patients. The study was performed in accordance with the Declaration of Helsinki.
Study patients and assessments
The REMAR study included female breast cancer patients ≥ 18 years, presenting with HR+ , HER2-negative, pN0/pN1mi/pN1, pT1-3, nuclear grade 1–3, and locally determined Ki-67 between 10 and 40%.
Ki-67 levels and tumor grades were evaluated both locally and centrally. The central evaluation was performed independent of the local evaluation. For central Ki-67 determination, tumor sections were sent blinded to OptiPath (S. A., Frankfurt, Germany). Ki-67 staining was performed with a ready-to-use antibody solution on Leica Bond machines (Leica Biosystems Division, Leica Microsystems Inc. Buffalo Grove, IL, United States) under standard conditions. The results were scanned with a slide scanner (3DHistech, Budapest, Hungary) and evaluated by image analysis using the ImmunoRatio software as a plugin in the ImageJ image processing package [22,23,24]. For each tumor, 3 representative image sections were quantified, and the mean value was calculated. 21-gene testing (Oncotype DX) was performed by Genomic Health (now part of Exact Sciences Corp., Redwood City, CA, United States) blinded for clinical and patient data. Ki-67 assessment and 21-gene testing were performed on samples from core needle biopsies.
Adjuvant treatment recommendations before and after the disclosure of the RS results were documented in a pre-specified case report form. Pre-RS, the recommended treatment was derived from histopathological (including local Ki-67) and clinical data, according to the St. Gallen consensus recommendation and the Arbeitsgemeinschaft Gynäkologische Onkologie e.V. (AGO) guidelines [25, 26]. Post-RS, treatment recommendations also incorporated the RS result. Central Ki-67 and central grading were not disclosed to the treating physicians throughout the study, and as such were not a consideration in treatment decision making.
Statistical analysis
Descriptive statistics were used to summarize histopathological and clinical characteristics and the RS results. Pearson product-moment correlation was used to compare the RS result with local and central Ki-67.
Results
Patients
The study included 567 eligible patients from 15 participating centers who were diagnosed between 9/2016 and 2/2019. Baseline patient and tumor characteristics are presented in Table 1. The median age was 57 years (range, 29–83). In total 395 (70%) patients with node negative (pN0), 8 patients (1%) were staged as pN1mi. Node positivity (pN1) was found in 164 (29%) patients at diagnosis. According to local grading, 438 patients (77%) had a grade 2 tumor. According to local Ki-67 evaluation, the mean Ki-67 level was 18.8% (the range was 10–40%, as dictated by the REMAR inclusion thresholds). According to the central Ki-67 evaluation, the mean Ki-67 level was 10.3% (range; < 1%–41%).
The median RS result was 17 (range, 0–61). The distribution of RS results using the cutoff values from the initial validation studies [6, 8, 9] demonstrated that 54%, 36%, and 10% of patients had RS results 0–17, 18–30, and 31–100, respectively. A similar analysis using an RS result cutoff value of 25 (as used in the Phase 3 validation studies TAILORx and RxPONDER [7, 10,11,12]) demonstrated that the majority of patients (81%) presented with a RS result between 0–25 and 19% with a RS result between 26 and 100 (Table 1).
Ki-67 assessment—Local vs. Central
The correlation between Ki-67 testing either locally or centrally (using a computerized count) is presented as Sankey flow diagram (Fig. 1). The locally assessed Ki-67 levels ranged from 10 to 40% (the inclusion threshold for REMAR) and used the routine 5% increment grouping, whereas the centrally determined Ki-67 is displayed as a continuous parameter and ranging from > 1 to 41%. The discrepancies between local and central Ki-67 evaluations were in both directions (i.e., the central Ki-67 was either lower or higher than the corresponding local evaluation). Notably, the concordance between local and central assessment was higher in low Ki-67 levels and decreased with increasing Ki-67 levels. Overall, the correlation between local and central Ki-67 levels was weak (r = 0.35). For 13 patients (2%), the difference in Ki-67 levels between local and central evaluations reflected a change in clinical risk and, thus, a shift in treatment recommendations if central Ki-67 would have been used to guide treatment recommendations instead of local Ki-67. These 13 patients included 12 with Ki-67local > 30% and Ki-67central ≤ 10% and 1 with Ki-67local ≤ 10% and Ki-67central > 30% (Fig. 1).
Sankey diagram displaying the correlation between Ki-67 determined centrally and locally (n = 562 patients with both local and central testing). The number of patients in each Ki-67 group is shown on both sides of the diagram. Local Ki-67 evaluations ranged from 10 to 40% and used routine grouping by 5% increments
Ki-67 and the RS results
The correlation between the RS result and Ki-67 (local vs. central) is presented (Fig. 2). The discrepancies between RS result and central Ki-67 (Fig. 2a) as well as between RS result and local Ki-67 (Fig. 2b), as seen in the Sankey diagrams were observed in both directions. Overall, the correlation between local Ki-67 and the RS result was weak (r = 0.3), as was the correlation between central Ki-67 and the RS result (r = 0.46).
There were cases with high RS results (26–100) and low local or central Ki-67 (0–10%), as well as cases with low RS results (0–11) and high Ki-67 levels. Of the patients with central Ki-67 of 0–10% (n = 344), 31 (9%) had RS results 26–100. Notably, of these 31 RS result 26–100 patients, 10 were ≤ 50, and 21 were > 50 years at diagnosis. Of the patients with central Ki-67 0–20% (n = 504), 73 (14%) had RS result 26–100. Of these 73 RS result 26–100 patients, 25 were ≤ 50, and 48 were > 50 years at diagnosis.
Changes in adjuvant treatment recommendations after the RS disclosure
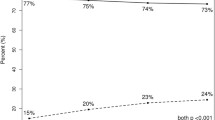
Absolute changes in adjuvant treatment recommendations following the disclosure of the RS result are presented for all patients (Fig. 3). Before disclosing the RS result, ET was recommended for 68% of patients and CET for 32% of patients. After disclosing the RS result, 76% of patients were recommended ET and only 24% were recommended CET (net absolute reduction in CET recommendation rate of 8%). In total, we observed a change in treatment recommendations for 190 women representing 34% of the entire population: 116 women with CET as initial therapy recommendation were transferred to ET only. In contrast, 74 individuals shifted from the initial ET group into the CET group.
Analysis of the changes in adjuvant treatment recommendations by RS result category demonstrated that treatment changes were aligned with the RS result. Among patients with RS result 0–25, treatment changes were noted in both directions but mostly involved de-escalation from CET to ET. The net effect of disclosing the RS result was an absolute reduction in CET recommendation rate of 22% (from 30 to 8%). In contrast, among patients with RS result 26–100, treatment changes were also in both directions, however, in all but one patient, these changes involved escalation from ET to CET. As expected, the net effect of disclosing the RS result in this group was an absolute increase in CET recommendation rate of 55% (from 37 to 92%) (Table 2).
With respect to nodal status (pN0 and pN1mi/pN1), changes of treatment recommendation in both directions (from ET to CET and from CET to ET) after disclosing the RS result were reported for a considerable proportion of the patients (31% and 40%, respectively) (Fig. 4a).
In node negative disease, changes in treatment recommendation from CET to ET occurred in 60 patients (15%) and from ET to CET in 62 individuals (16%), respectively. Thus, in absolute numbers before and after disclosing the RS results, 76% of patients were recommended ET and in 24% CET was recommended. However, in patients presenting with pN1mi/pN1 disease, representing an increased risk of recurrence, changes from CET to ET were more common than from ET to CET resulting in an absolute reduction in CET recommendation of 26% (from 50 to 24%) (Fig. 4a).
Analysis of the changes in adjuvant treatment recommendations was also performed by central Ki-67 levels using 10% as the cutoff value (Fig. 4b). This analysis examined patients with central Ki-67 ≤ 10% (n = 346) and patients with central Ki-67 > 10% (n = 216) separately. In a considerable number of patients (up to 45%), we observed recommendation changes. In patients with Ki-67 ≤ 10%, before disclosing the RS result, 74% were recommended ET and 26% were recommended CET. These recommendations were subject to change in 93 patients (27%). As de-escalation of treatment recommendation from CET to ET was more common than escalation from ET to CET, the absolute reduction in CET recommendation was 12% (from 26 to 14%). In patients with Ki-67 > 10%, before disclosing the RS result, 59% of patients were assigned to ET and CET was recommended for 41%. Changes in treatment recommendations were noted in 96 patients (45%). In 49 (23%) women, we observed a de-escalation from CET to ET and an escalation form ET to CET was observed in 47 (22%) women. The result was a net absolute reduction in CET recommendation rate of only 1% (from 41 to 40%).
Discussion
The current study demonstrated a weak correlation between local and central Ki-67 assessment and between Ki-67 levels and the RS results. These findings are consistent with prior reports investigating Ki-67 reproducibility and inter/intra-observer variability [16,17,18], including findings from the prospective randomized phase 3 Plan-B study [25,26,27,28,29,30,31,32,33].
The current study also revealed changes in treatment recommendations that are aligned with the RS result (i.e., treatment de-escalation in RS result 0–25 patients and treatment escalation in RS result 26–100 patients), in a considerable proportion of the cohort overall (34%) and in pN0 and pN1mi/N1 patients separately (31% and 40%, respectively) with an overall net reduction in CET use. The decision-impact results are consistent with a large body of prospective data from studies conducted worldwide, which also showed such an impact on a substantial proportion of patients alongside an overall net reduction in CT use (reviewed in [34]). To the best of our knowledge, this is the first study to evaluate the impact of knowing the RS by central Ki-67 results. Most RS prospective decision-impact studies did not report the Ki-67 results of the study population. Notably, a Spanish study by Albanell and colleagues, in which the Ki-67 distribution (presumably locally determined) was reported, addressed the role of Ki-67 in treatment decision making through a univariate analysis that evaluated the association between clinicopathological variables and the likelihood of change in adjuvant treatment recommendations after knowing the RS result. The analysis demonstrated that high Ki-67 (≥ 20%) was significantly associated with a greater chance of ET to CET change, whereas a change from CET to ET was not significantly associated with Ki-67 values. Also, this univariate analysis was limited by a relatively small sample size in some of the subgroups [35].
In both the current real-world analysis, and the translational program of the Plan-B study (2274 patients with HR + HER2-negative pN0-1 early-stage breast cancer), close to a tenth of patients with low risk of recurrence according to Ki-67 level were reclassified as high risk according to the RS result (9% and 8%, respectively) [29]. A PlanB analysis which evaluated DFS by centrally determined Ki-67 groups (low: 0–10%, n = 790; intermediate: > 10% and < 40%, n = 965) and the RS result (RS: 0–11, 12–25, 26–100), showed that within the intermediate Ki-67 group, the RS was highly prognostic, whereas within the low Ki-67 group the impact of the RS was not observed. However, only 33 events occurred among all low Ki-67 patients, and only 66 patients (8.4%) had high RS [21].
Data from both the REMAR and Plan-B trials suggest that applying a Ki-67 threshold for identifying patients at intermediate risk with limiting access to the 21-gene assay might face a chance for undertreatment for a significant number of patients. Such undertreatment is of particularly relevance for young women under the age of 50, which represent about one third of our study population. As evidence suggests, these patients have a higher risk of recurrence per se. The findings also suggest that nodal status is only a weak prognosticator, as both patient subgroups (pN0 vs. pN1mi/N1) benefited from adjuvant CET [7, 10].
Following the disclosure of the RS, treatment recommendation changes were reported in both directions (escalation and de-escalation of treatment) in alignment with the RS results. Substantial change rates were observed across all the subgroups defined by nodal status, and Ki-67 level suggesting a need for a reliable clinically relevant assessment to prevent both under as well as overtreatment. Overall, the net result was a reduction in CET use; however, in node-negative disease, these treatment recommendation changes balanced out without substantial changes, at all. Notably, the decision impact of the 21-gene assay was more pronounced in the pN1mi/N1 population. More patients were recommended CET compared to the node-negative population, whereas after the assay, the proportion of patients assigned to decreased and was comparable to the use in the pN0 population (24%). Consistent with the initial validation studies in this population, as well as with the more recent phase 3 RxPONDER data demonstrating very good clinical outcomes for node-positive patients presenting with a RS result ≤ 25 treated by ET alone [7].
The REMAR results and other real-world data suggest that Ki-67 levels should not be used as eligibility criteria for initiating a 21-gene testing. For example, initially, the FDA approved abemaciclib (a CDK4/6 inhibitor) plus ET as adjuvant treatment for patients with a high-risk of recurrence in HR + HER2-negative, node-positive EBC, and Ki-67 ≥ 20% based on results from the MonarchE trial [36]. However, in 3/2021, the FDA resigned from recommending the Ki-67 level at all, based on updated data from this trial showing similar proportional invasive disease-free survival (iDFS) benefit regardless of Ki-67 status [37, 38].
Notably, the recent prospective phase 3 trial, WSG-ADAPT in HR + /HER2-negative disease suggests that Ki-67 as a dynamic biomarker after short-term neoadjuvant ET in combination with the RS result might be an additional biomarker to guide adjuvant systemic therapy decisions in pN0-1 HR + HER2-negative breast cancer. Patients received adjuvant ET if their RS result was 0–11 (control arm), or if the RS result was 12–25 and they responded to preoperative ET (experimental arm). Patients with RS result 12–25 disclosing no response to preoperative ET received CET. The ADAPT data demonstrated that the feasibility of this approach demonstrating that iDFS in the experimental arm was noninferior to the control arm. The 5-year iDFS was 92.6% (95% confidence interval [CI], 90.8–94.0%) in the experimental arm vs. 93.9% (95% CI, 91.8–95.4%) in the control arm [39, 40]. The ongoing WSG-ADAPT cycle study, a prospective, randomized phase 3 trial, investigates whether patients with HR + HER2-negative early-stage breast cancer with intermediate risk based on the RS result and response to preoperative ET (i.e., RS result ≤ 25 and Ki-67 > 10% post-induction, RS result > 25 and Ki-67 ≤ 10% post-induction in c/pN0-1 patients, or RS result ≤ 25 and Ki-67 ≤ 10% in c/pN2-3 patients) obtain additional benefit from 2 years of ribociclib (a CDK4/6 inhibitor) combined with ET compared to CT followed by ET. Interim analysis of this trial further supported the practice of combining the RS result and post-induction dynamic changes of Ki-67 for guiding adjuvant treatment and suggested that adding ovarian function suppression to tamoxifen or aromatase inhibitors (AI) improves response to ET in premenopausal patients, resulting in a response rate comparable to that of AI-treated postmenopausal patients [41].
The current study is limited by the methodology of decision-impact registries. Additionally, REMAR focused on correlations and decision-impact description and was not designed to record clinical outcome data.
Conclusions
In conclusion, our data demonstrate in addition to the existing evidence that Ki-67 results are not validly reproducible and demonstrate a weak correlation with the RS results. Thus, the Ki-67 and the RS result are not interchangeable. Finally, the recent evidence is suggesting that Ki-67 levels should not fence the decision to indicate use of the 21-gene assay as we demonstrate like others that this test is prognostic and predictive for driving treatment decisions in luminal EBC. For the future, it is noteworthy to point out that we have to educate ourselves to leave out static considerations tools for treatment decision behind and welcome dynamic tools such as Ki-67 measurement after short-term exposure of ET to monitor endocrine sensitivity to ET. Hence, we have to welcome dynamic parameters such as dynamic Ki-67 testing as introduced in the WSG ADACT Cycle trial. The RS results provide a valuable addition to the classic risk assessment and treatment decision factors as a tool to individualize treatment decisions. Nodal status and Ki-67 alone should not be used as a gatekeeper for testing eligibility, in order to avoid under and overtreatment.
Data availability
The dataset used in the current study is available from the corresponding author upon reasonable request.
References
Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63(1):181–187
Ly A, Lester SC, Dillon D (2012) Prognostic factors for patients with breast cancer: traditional and new. Surg Pathol Clin 5(3):775–785. https://doi.org/10.1016/j.path.2012.06.010
McGuire WL, Tandon AK, Allred DC, Chamness GC, Clark GM (1990) How to use prognostic factors in axillary node-negative breast cancer patients. J Natl Cancer Inst 82(12):1006–1015. https://doi.org/10.1093/jnci/82.12.1006
Tandon AK, Clark GM, Chamness GC, Chirgwin JM, McGuire WL (1990) Cathepsin D and prognosis in breast cancer. N Engl J Med 322(5):297–302. https://doi.org/10.1056/NEJM199002013220504
Wong WW, Vijayakumar S, Weichselbaum RR (1992) Prognostic indicators in node-negative early stage breast cancer. Am J Med 92(5):539–548. https://doi.org/10.1016/0002-9343(92)90751-v
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65. https://doi.org/10.1016/S1470-2045(09)70314-6
Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, Lin NU, Perez EA, Goldstein LJ, Chia SKL, Dhesy-Thind S, Rastogi P, Alba E, Delaloge S, Martin M, Kelly CM, Ruiz-Borrego M, Gil-Gil M, Arce-Salinas CH, Brain EGC, Lee ES, Pierga JY, Bermejo B, Ramos-Vazquez M, Jung KH, Ferrero JM, Schott AF, Shak S, Sharma P, Lew DL, Miao J, Tripathy D, Pusztai L, Hortobagyi GN (2021) 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 385(25):2336–2347. https://doi.org/10.1056/NEJMoa2108873
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. https://doi.org/10.1056/NEJMoa041588
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734. https://doi.org/10.1200/JCO.2005.04.7985
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121. https://doi.org/10.1056/NEJMoa1804710
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373(21):2005–2014. https://doi.org/10.1056/NEJMoa1510764
Sparano JA, Gray RJ, Ravdin PM, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Keane MM, Moreno HLG, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr (2019) Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med 380(25):2395–2405. https://doi.org/10.1056/NEJMoa1904819
Hyams DM, Schuur E, Angel Aristizabal J, Bargallo Rocha JE, Cabello C, Elizalde R, Garcia-Estevez L, Gomez HL, Katz A, Nunez De Pierro A (2017) Selecting postoperative adjuvant systemic therapy for early stage breast cancer: a critical assessment of commercially available gene expression assays. J Surg Oncol 115(6):647–662. https://doi.org/10.1002/jso.24561
Markopoulos C, Hyams DM, Gomez HL, Harries M, Nakamura S, Traina T, Katz A (2020) Multigene assays in early breast cancer: insights from recent phase 3 studies. Eur J Surg Oncol. https://doi.org/10.1016/j.ejso.2019.10.019
Markopoulos C, van de Velde C, Zarca D, Ozmen V, Masetti R (2017) Clinical evidence supporting genomic tests in early breast cancer: Do all genomic tests provide the same information? Eur J Surg Oncol 43(5):909–920. https://doi.org/10.1016/j.ejso.2016.08.012
Focke CM, Burger H, van Diest PJ, Finsterbusch K, Glaser D, Korsching E, Decker T, German Breast Screening Pathology Initiative (2017) Interlaboratory variability of Ki67 staining in breast cancer. Eur J Cancer 84:219–227. https://doi.org/10.1016/j.ejca.2017.07.041
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM, Gown AM, Symmans WF, Piper T, Mehl E, Enos RA, Hayes DF, Dowsett M, Nielsen TO, International Ki67 in Breast Cancer Working Group of the Breast International Group and North American Breast Cancer Group (2013) An international ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906. https://doi.org/10.1093/jnci/djt306
Varga Z, Diebold J, Dommann-Scherrer C, Frick H, Kaup D, Noske A, Obermann E, Ohlschlegel C, Padberg B, Rakozy C, Sancho Oliver S, Schobinger-Clement S, Schreiber-Facklam H, Singer G, Tapia C, Wagner U, Mastropasqua MG, Viale G, Lehr HA (2012) How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the swiss working group of breast-and gynecopathologists. PLoS ONE 7(5):e37379. https://doi.org/10.1371/journal.pone.0037379
NCCN clinical practice guidelines in oncology. Breast Cancer. Version 4.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed, Sep 23, 2023.
Nielsen TO, Leung SCY, Rimm DL, Dodson A, Acs B, Badve S, Denkert C, Ellis MJ, Fineberg S, Flowers M, Kreipe HH, Laenkholm AV, Pan H, Penault-Llorca FM, Polley MY, Salgado R, Smith IE, Sugie T, Bartlett JMS, McShane LM, Dowsett M, Hayes DF (2020) Assessment of Ki67 in breast cancer: updated recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst 113(7):808–819. https://doi.org/10.1093/jnci/djaa201
Nitz U, Gluz O, Christgen M, Kates RE, Clemens M, Malter W, Nuding B, Aktas B, Kuemmel S, Reimer T, Stefek A, Lorenz-Salehi F, Krabisch P, Just M, Augustin D, Liedtke C, Chao C, Shak S, Wuerstlein R, Kreipe HH, Harbeck N (2017) Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: Five-year data from the prospective, randomised phase 3 West German study group (WSG) PlanB trial. Breast Cancer Res Treat 165(3):573–583. https://doi.org/10.1007/s10549-017-4358-6
Konsti J, Lundin M, Joensuu H, Lehtimaki T, Sihto H, Holli K, Turpeenniemi-Hujanen T, Kataja V, Sailas L, Isola J, Lundin J (2011) Development and evaluation of a virtual microscopy application for automated assessment of Ki-67 expression in breast cancer. BMC Clin Pathol 11:3. https://doi.org/10.1186/1472-6890-11-3
Tuominen VJ, Ruotoistenmaki S, Viitanen A, Jumppanen M, Isola J (2010) ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res 12(4):R56. https://doi.org/10.1186/bcr2615
Yeo MK, Kim HE, Kim SH, Chae BJ, Song BJ, Lee A (2017) Clinical usefulness of the free web-based image analysis application ImmunoRatio for assessment of Ki-67 labelling index in breast cancer. J Clin Pathol 70(8):715–719. https://doi.org/10.1136/jclinpath-2016-204162
Vera-Badillo FE, Chang MC, Kuruzar G, Ocana A, Templeton AJ, Seruga B, Goldstein R, Bedard PL, Tannock IF, Amir E (2015) Association between androgen receptor expression, Ki-67 and the 21-gene recurrence score in non-metastatic, lymph node-negative, estrogen receptor-positive and HER2-negative breast cancer. J Clin Pathol 68(10):839–843. https://doi.org/10.1136/jclinpath-2015-203012
Walter VP, Taran FA, Wallwiener M, Bauer A, Grischke EM, Walter CB, Hahn M, Brucker SY, Hartkopf AD (2020) Distribution of the 21-gene breast recurrence score in patients with primary breast cancer in Germany. Geburtshilfe Frauenheilkd 80(6):619–627. https://doi.org/10.1055/a-1111-8734
Aktas B, Bankfalvi A, Heubner M, Kimmig R, Kasimir-Bauer S (2013) Evaluation and correlation of risk recurrence in early breast cancer assessed by oncotype DX((R)), clinicopathological markers and tumor cell dissemination in the blood and bone marrow. Mol Clin Oncol 1(6):1049–1054. https://doi.org/10.3892/mco.2013.174
Albanell J, Svedman C, Gligorov J, Holt SD, Bertelli G, Blohmer JU, Rouzier R, Lluch A, Eiermann W (2016) Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur J Cancer 66:104–113. https://doi.org/10.1016/j.ejca.2016.06.027
Gluz O, Nitz UA, Christgen M, Kates RE, Shak S, Clemens M, Kraemer S, Aktas B, Kuemmel S, Reimer T, Kusche M, Heyl V, Lorenz-Salehi F, Just M, Hofmann D, Degenhardt T, Liedtke C, Svedman C, Wuerstlein R, Kreipe HH, Harbeck N (2016) West German study group phase III PlanB trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol 34(20):2341–2349. https://doi.org/10.1200/JCO.2015.63.5383
Sahebjam S, Aloyz R, Pilavdzic D, Brisson ML, Ferrario C, Bouganim N, Cohen V, Miller WH Jr, Panasci LC (2011) Ki 67 is a major, but not the sole determinant of oncotype Dx recurrence score. Br J Cancer 105(9):1342–1345. https://doi.org/10.1038/bjc.2011.402
Tan AC, Li BT, Nahar K, Danieletto S, Fong ES, Currer T, Parasyn A, Middleton P, Wong H, Smart D, Rutovitz JJ, McCloud P, Hughes TM, Marx GM (2018) Correlating Ki67 and other prognostic markers with oncotype DX recurrence score in early estrogen receptor-positive breast cancer. Asia Pac J Clin Oncol 14(2):e161–e166. https://doi.org/10.1111/ajco.12779
Thakur SS, Li H, Chan AMY, Tudor R, Bigras G, Morris D, Enwere EK, Yang H (2018) The use of automated Ki67 analysis to predict oncotype DX risk-of-recurrence categories in early-stage breast cancer. PLoS ONE 13(1):e0188983. https://doi.org/10.1371/journal.pone.0188983
Williams DJ, Cohen C, Darrow M, Page AJ, Chastain B, Adams AL (2011) Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol 19(5):431–436. https://doi.org/10.1097/PAI.0b013e318206d23d
Siow ZR, De Boer RH, Lindeman GJ, Mann GB (2018) Spotlight on the utility of the oncotype DX((R)) breast cancer assay. Int J Womens Health 10:89–100. https://doi.org/10.2147/IJWH.S124520
Albanell J, González A, Ruiz-Borrego M, Alba E, García-Saenz JA, Corominas JM, Burgues O, Furio V, Rojo A, Palacios J, Bermejo B, Martínez-García M, Limon ML, Muñoz AS, Martín M, Tusquets I, Rojo F, Colomer R, Faull I, Lluch, (2012) A Prospective transGEICAM study of the impact of the 21-gene recurrence score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol 23(3):625–631. https://doi.org/10.1093/annonc/mdr278
Royce M, Osgood C, Mulkey F, Bloomquist E, Pierce WF, Roy A, Kalavar S, Ghosh S, Philip R, Rizvi F, Mixter BD, Tang S, Pazdur R, Beaver JA, Amiri-Kordestani L (2022) FDA approval summary: Abemaciclib with endocrine therapy for high-risk early breast cancer. J Clin Oncol 40(11):1155–1162. https://doi.org/10.1200/JCO.21.02742
Abemaciclib [Package Insert]. Indianapolis, IN: Eli Lilly and Company; 2023.
Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, Campone M, Neven P, Huang CS, Huober J, Jaliffe GG, Cicin I, Tolaney SM, Goetz MP, Rugo HS, Senkus E, Testa L, Del Mastro L, Shimizu C, Wei R, Shahir A, Munoz M, San Antonio B, Andre V, Harbeck N, Martin M, monarch ECM, (2023) Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol 24(1):77–90. https://doi.org/10.1016/S1470-2045(22)00694-5
Nitz U, Gluz O, Kreipe HH, Christgen M, Kuemmel S, Baehner FL, Shak S, Aktas B, Braun M, Ludtke-Heckenkamp K, Forstbauer H, Grischke EM, Nuding B, Darsow M, Schumacher C, Krauss K, Malter W, Thill M, Warm M, Wuerstlein R, Kates RE, Harbeck N (2020) The run-in phase of the prospective WSG-ADAPT HR+/HER2-trial demonstrates the feasibility of a study design combining static and dynamic biomarker assessments for individualized therapy in early breast cancer. Ther Adv Med Oncol. https://doi.org/10.1177/1758835920973130
Nitz UA, Gluz O, Kummel S, Christgen M, Braun M, Aktas B, Ludtke-Heckenkamp K, Forstbauer H, Grischke EM, Schumacher C, Darsow M, Krauss K, Nuding B, Thill M, Potenberg J, Uleer C, Warm M, Fischer HH, Malter W, Hauptmann M, Kates RE, Graser M, Wurstlein R, Shak S, Baehner F, Kreipe HH, Harbeck N (2022) Endocrine therapy response and 21-gene expression assay for therapy guidance in HR+/HER2-early breast cancer. J Clin Oncol 40(23):2557–2567. https://doi.org/10.1200/JCO.21.02759
Gluz O, Nitz U, Christgen M, Kümmel S, Braun M, Thill MA, B., Wimberger P, Luedtke-Heckenkamp K, Zaiss MW, M., Schumacher C, Schem C, Forstbauer H, Krauss KG, M., Hartkopf A, Kates R, Eulenburg C, Schmid PK, H., Harbeck N (2022) Impact of age, recurrence score (RS) and ovarian function suppression (OFS) on endocrine response to short preoperative endocrine therapy (ET): analysis of ADAPT and ADAPTcycle trials. Presented at the ESMO Congress 2022, 9-13 September 2022, Paris, France
Acknowledgements
The authors thank Avital Bareket-Samish, PhD, for medical editing support, which was funded by Exact Sciences.
Funding
Agaplesion Markus Hospital, Frankfurt am Main, Germany, was the regulatory Sponsor of this study. This work was supported by a clinical research grant from Exact Sciences. Exact Sciences had no role in study design, study data collection, analysis, and interpretation, and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
C. Jackisch and M. Thill contributed to study conception and design. Data collection was performed by C. Jackisch, L. Anastasiadou, S, Aulmann, A. Argyriadis, V, Möbus, C. Solbach, P. Baier, D. Giesecke, S. Ackermann, E. Schulmeyer, B. Gabriel, D. Mosch, S. Buchen, E. Krapfl, U. Hurst, M. Vescia, H. Tesch, and M. Thill. Data analysis was performed by C. Jackisch, A. Argyriadis, and M. Thill. The first draft of the manuscript was written by C. Jackisch, A. Argyriadis, and M. Thill. All authors commented on subsequent versions of the manuscript and provided critical feedback. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Financial interests: C. Jackisch reports participating in advisory boards for Roche, AstraZeneca, Pfizer, Celgene, Lilly, and Exact Sciences; being a speaker and chairman for educational events for Roche, AstraZeneca, Pfizer, Celgene, Exact Sciences, Lilly, and Pierre-Fabré; and receiving research funding from Novartis, Exact Sciences, and Roche. M. Thill reports participating in advisory boards for Agendia, Amgen, AstraZeneca, Aurikamed, Becton/Dickinson, Biom ‘Up, ClearCut, Clovis, Daiichi Sankyo, Eisai, Exact Sciences, Gilead Science, Grünenthal, GSK, Lilly, MSD, Norgine, Neodynamics, Novartis, Onkowissen, Organon, Pfizer, pfm Medical, Pierre-Fabre, Roche, RTI Surgical, Seagen, Sirius Pintuition, and Sysmex; receiving manuscript support from Amgen, ClearCut, Clovis, pfm medical, Roche, and Servier; receiving travel support from Amgen, Art Tempi, AstraZeneca, Clearcut, Clovis, Connect Medica, Daiichi Sankyo, Eisai, Exact Sciences, Hexal, I-Med-Institute, Lilly, MCI, Medtronic, MSD, Norgine, Novartis, Pfizer, pfm Medical, Roche, RTI Surgical, and Seagen; congress support from Amgen, AstraZeneca, Celgene, Daiichi Sanyko, Hexal, Neodynamics, Novartis, Pfizer, and Roche; lecture honoraria from Amgen, Art Tempi, AstraZeneca, Clovis, Connect Medica, Eisai, Exact Sciences, Gilead Science, Hexal, I-Med-Institute, Jörg Eickeler, Lilly, MCI, Medtronic, MSD, Novartis, Omniamed, Onkowissen, Pfizer, pfm Medical, Roche, RTI Surgical, Seagen, Sysmex, Vifor, and Viatris; and trial funding from Endomag, and Exact Sciences. The remaining authors declare no competing financial or non-financial interests.
Ethical approval
The study protocol was approved by the responsible Ethics Committee at the Board of Physicians in Hessen (study number FF48/2015) and informed consent was obtained from all participating patients. The study has been carried out in accordance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jackisch, C., Anastasiadou, L., Aulmann, S. et al. The REMAR (Rhein-Main-Registry) real-world study: prospective evaluation of the 21-gene breast recurrence score® assay in addition to Ki-67 for adjuvant treatment decisions in early-stage breast cancer. Breast Cancer Res Treat 207, 263–274 (2024). https://doi.org/10.1007/s10549-024-07390-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07390-y