Abstract
Purpose
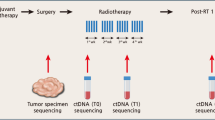
Circulating cell-free DNA (cfDNA) is a promising biomarker for predicting treatment response and disease outcomes in Breast Cancer (BC) patients undergoing neoadjuvant chemotherapy (NAC). To determine if cfDNA originates from tumors, matching tumor and cfDNA gene mutations are necessary, often requiring tumor DNA sequencing. We assessed plasma cfDNA integrity by measuring concentrations and ratios of larger-to-smaller Alu DNA fractions as a potential biomarker, eliminating the need for prior tumor sequencing.
Methods
We included patients with localized and/or locally advanced BC receiving standard NAC alone or in combination with immunotherapy and/or anti-HER2 targeted therapy. Blood samples were collected before treatment, every 2 weeks during treatment, and before surgery.
Results
Of the 38 evaluated patients, only 28 completed the protocol and underwent surgery after NAC. Seven patients (25%) achieved a pathologic complete response (pCR). We found that cfDNA integrity (cfDNAI) levels at 15 days after starting NAC were significantly higher in patients who achieved pCR (p = 0.045) and correlated significantly with Disease-Free Survival (DFS) in univariate analysis (p = 0.0371).
Conclusions
Evaluation of cfDNAI 2 weeks after NAC initiation appears to be an early biomarker for tumor pCR and DFS. Measuring Alu fragments of different lengths may replace techniques requiring prior tumor sequencing to measure ctDNA, reducing costs and complexity of cfDNA serial measurements in BC patients undergoing NAC.



Similar content being viewed by others
Data availability
Data will be made available under request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Asaoka M, Gandhi S, Ishikawa T, Takabe K (2020) Neoadjuvant chemotherapy for breast cancer: past, present, and future. Breast Cancer Basic Clin Res 14:1–8. https://doi.org/10.1177/1178223420980377
Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R (2001) DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Can Res 61(4):1659–1665
Burnham P, Kim MS, Agbor-Enoh S, Luikart H, Valantine HA, Khush KK, De Vlaminck I (2016) Single-stranded DNA library preparation uncovers the origin and diversity of ultrashort cell-free DNA in plasma. Sci Rep 6:27859
Bryzgunova OE, Laktionov PP (2014) Generation of blood circulating DNAs: sources, features of structure and circulation. Biochem (Moscow) Suppl Series B: Biomed Chem 8(3):203–219. https://doi.org/10.18097/PBMC20156104409
Aucamp J, Bronkhorst AJ, Badenhorst CPS, Pretorius PJ (2018) The diverse origins of circulating cell-free DNA in the human body: a critical re-evaluation of the literature. Biol Rev 93:1649–1683. https://doi.org/10.1111/brv.12413
Delgado PO, Alves BCA, Gehrke FS, Kuniyoshi RK, Wroclavski ML, Giglio AD, Fonseca FLA (2013) Characterization of cell-free circulating DNA in plasma in patients with prostate cancer. Tumour Biol 34:983–986. https://doi.org/10.1007/s13277-012-0634-6
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7(2):99–109
van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228. https://doi.org/10.1038/nrm.2017.125
Lögters T, Margraf S, Altrichter J, Cinatl J, Mitzner S, Windolf J, Scholz M (2009) Clinical value of neutrophil extracellular traps. Med Microbiol Immunol 198:211–219. https://doi.org/10.1007/s00430-009-0121-x
Telekes A, Horváth A (2022) The role of cell-free DNA in cancer treatment decision making. Cancers 14(24):6115. https://doi.org/10.3390/cancers14246115
Umetani N, Giuliano AE, Hiramatsu SH, Amersi F, Nakagawa T, Martino S, Hoon DSB (2006) Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol 24(26):4270–4276
Arko-Boham B, Aryee NA, Blay RM, Owusu EDA, Tagoe EA, Doris Shackie ES, Debrah AB, Adu-Aryee NA (2019) Circulating cell-free DNA integrity as a diagnostic and prognostic marker for breast and prostate cancers. Cancer Genet. https://doi.org/10.1016/j.cancergen.2019.04.062
Nair MG, Ramesh RS, Naidu CM, Mavatkar AD, Snijesh VP, Ramamurthy V, Somashekaraiah VM, Anupama CE, Raghunathan K, Panigrahi A, Das M, Dhar SK, Prabhu JS (2023) Estimation of ALU repetitive elements in plasma as a cost-effective liquid biopsy tool for disease prognosis in breast cancer. Cancers 15(4):1054. https://doi.org/10.3390/cancers15041054
Buono G, Gerratana L, Bulfoni M, Provinciali N, Basile D, Giuliano M, Corvaja C, Arpino G, Del Mastro L, De Placido S, De Laurentiis M, Cristofanilli M, Puglisi F (2019) Circulating tumor DNA analysis in breast cancer: is it ready for prime-time? Cancer Treat Rev 73:73–83. https://doi.org/10.1016/j.ctrv.2019.01.004
Elhelaly R, Effat N, Hegazy MAE, Abdelwahab K, Hamdy O, Abo Hashem EM, Elzehery RR (2022) Circulating cell free DNA and DNA integrity index as discriminating tools between breast cancer and benign breast disease. Asian Pac J Cancer Prev 23(2):545–552. https://doi.org/10.31557/APJCP.2022.23.2.545
Salimi M, Sedaghati Burkhani S (2019) Integrity and quantity evaluation of plasma cell-free DNA in triple negative breast cancer. Avicenna J Med Biotechnol 11(4):334–338
Sobhani N, Generali D, Zanconati F, Bortul M, Scaggiante B (2018) Cell-free DNA integrity for the monitoring of breast cancer: future perspectives? World J Clin Oncol 9(2):26–32. https://doi.org/10.5306/wjco.v9.i2.26
Serpa Neto A, Wroclawski ML, Pinto JLF, Marsicano SR, Delgado PO, Coelho PG, Moreno R, Vilas Boas VA, Azzalis LA, Junqueira VBC, Giglio AD, Fonseca FLA (2012) Methodological standardization for the extraction of free DNA in plasma of peripheral blood. J Cancer Sci Ther. https://doi.org/10.4172/1948-5956.S5-005
Iqbal S, Vishnubhatla S, Raina V, Sharma S, Gogia A, Deo SS, Mathur S, Shukla N (2015) Circulating cell-free DNA and its integrity as a prognostic marker for breast cancer. Springerplus 4:265. https://doi.org/10.1186/s40064-015-1071-y
Adusei E, Ahenkorah J, Adu-Aryee NA, Adutwum-Ofosu KK, Tagoe EA, Koney NK, Nkansah E, Aryee NA, Blay RM, Hottor BA, Clegg-Lamptey JN, Arko-Boham B (2021) Reduced serum circulation of cell-free DNA following chemotherapy in breast cancer patients. Med Sci 9(2):37. https://doi.org/10.3390/medsci9020037
Wang W, Zhang W, Su L, Sang J, Wang S, Yao Y (2019) Plasma cell-free DNA integrity: a potential biomarker to monitor the response of breast cancer to neoadjuvant chemotherapy. Transl Cancer Res. 8(4):1531–1539. https://doi.org/10.21037/tcr.2019.08.05
Cirmena G, Ferrando L, Ravera F, Garuti A, Dameri M, Gallo M, Barbero V, Ferrando F, Del Mastro L, Garlaschi A, Friedman D, Fregatti P, Ballestrero A, Zoppoli G (2022) Plasma cell-free DNA integrity assessed by automated electrophoresis predicts the achievement of pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer. JCO Precis Oncol. https://doi.org/10.1200/PO.21.00198
Lehner J, Stötzer OJ, Fersching DMI, Nagel D, Holdenrieder S (2013) Plasma DNA integrity indicates response to neoadjuvant chemotherapy in patients with locally confined breast cancer. Int J Clin Pharmacol Ther 51(01):59–62. https://doi.org/10.5414/cpp51059
Cell-free DNA fragmentomics: A promising biomarker for diagnosis, prognosis and prediction of response in breast Cancer. https://agris.fao.org/agris-search/search.do?recordID=DJ2023085113 Accessed 3 Jun 2023
Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, Tin A, Salari R, Shchegrova S, Pawar H, Delson AL, DeMichele A, Liu MC, Chien AJ, Tripathy D, Asare S, Lin CJ, Billings P, Aleshin A, Sethi H, Louie M, Zimmermann B, Esserman LJ, van.t Veer LJ (2021) Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol 32(2):229–239. https://doi.org/10.1016/j.annonc.2020.11.007
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization was performed by Fernando Luiz Affonso Fonseca, Lilian A. do R. Barros, and Auro del Giglio; methodology and validation were performed by Beatriz da Costa Aguiar Alves; formal analysis was performed by Camila Giro; investigation was performed by Alayne M. T. D. Yamada and Felipe José S. M. Cruz; resources were obtained by Auro del Giglio and Fernando Luiz Affonso Fonseca; the first draft of the manuscript was written by Auro del Giglio and Beatriz da Costa Aguiar Alves. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Centro Universitário FMABC/ IBCC (protocol code 32158620.0.0000.0072, approved on July 2020) and we registered on ClinicalTrials.gov under the identifier NCT05050890. Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giro, C., Yamada, A.M.T.D., Cruz, F.J.S.M. et al. Measuring cfDNA integrity as a biomarker for predicting neoadjuvant chemotherapy response in breast cancer patients: a pilot study. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07366-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10549-024-07366-y