Abstract
Purpose
The importance of a TP53 mutation has been demonstrated in several tumor types, including breast cancer (BC). However, the accuracy of p53 protein expression as a predictor of gene mutation has not been well studied in BC. Therefore, we evaluated p53 protein expression associated with TP53 mutations in breast cancers from 64 patients.
Methods
TP53 mutation was examined using next-generation sequencing (NGS). p53 protein expression was examined using immunohistochemistry (IHC).
Results
Among the 64 BCs, 55% demonstrated abnormal expression patterns including 27% overexpression, 22% null, 6% equivocal with 45% having a wild-type pattern. A TP53 mutation was present in 53% (34/64) of tumors including 30% (19/64) demonstrating a missense mutation, 11% (7/64) with a frameshift mutation, 11% (7/64) with a nonsense mutation, and 3% (1/64) with a splice site mutation. Abnormal expression of p53 protein was present in 33 of 34 (97%) tumors carrying a TP53 mutation; conversely, a wild-type pattern was present in 28 of 30 (93%) tumors without a detectable mutation (p < 0.0001). The majority of BCs with a p53 IHC overexpression pattern (15/17, 88%) contained a missense TP53 mutation; while the majority of BCs with a null pattern (12/14, 86%) contained a truncating mutation (p < 0.0001). The BCs with a null pattern are associated with a high Nottingham histological grade and a triple-negative phenotype when compared to those demonstrating overexpression (p < 0.05).
Conclusion
These findings suggest that p53 IHC can be a potential surrogate for TP53 mutations in BC. Different p53 expression patterns may correlate with specific TP53 genetic mutations in BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
TP53 is the most frequently mutated gene identified in most cancer types [1]. p53 protein is a regulatory protein referred as “the guardian of the genome,” involved in multiple physiologic activities including modulating the cell cycle, apoptosis, and genomic stability [2]. Mutations in the TP53 gene affect tumor suppressor function and confer the oncogenic properties of p53 protein [2]. Approximately 30% of breast cancers (BC) harbor a TP53 mutation [3]. Studies report that a TP53 mutation is an independent prognostic marker predicting poor prognosis in BC and to a lesser extent in p53 protein expression [4, 5].
From a practical standpoint, the clinical applicability of p53 expression for patients with BC has yet to be confirmed. The correlation between p53 expression and TP53 mutation is currently suboptimal [6, 7]. Besides the biological instability of p53 protein with variable detecting assays, the lack of consensus on interpretation is a major issue. The majority of clinical laboratories utilize immunohistochemistry (IHC) to examine the p53 protein expression in breast tumor specimens. The assessment of p53 IHC staining is predominantly based on protein overexpression, and reported as “positive” or “negative,” or provided as a “percentage.” However, accumulating studies have shown that other IHC patterns, such as a complete absence or cytoplasmic expression, can also indicate a TP53 mutation in several cancer types [7,8,9]. Optimizing the interpretation of p53 IHC staining is crucial for patient management of BC; therefore, we aim to improve the correlation between p53 IHC expression and TP53 mutation status, with appropriate interpretation.
Methods
Invasive breast carcinoma samples
Patients diagnosed with invasive breast carcinoma between 2014 and 2022 at our institution whose tumors underwent next-generation sequencing (NGS) were identified. For these patients, we used samples of biopsy or surgical excision specimens from the primary or metastatic tumor. Patient age, tumor size, pathologic T, N and M stage, and history of neoadjuvant chemotherapy (NACT) were collected from slide review and the patients’ medical record after institutional IRB approval.
Histology
All stained slides were reviewed independently by two pathologists (XH and SAA) and the pathologic characteristics were affirmed, including histologic grade, histologic type, and predictive marker status. The American Society of Clinical Oncology/College of American Pathologists guideline recommendations [10,11,12] were used as references to categorized estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status as part of the routine pathologic evaluation. Tumors with ER-low positivity (1–10%) were considered as ER-positive in this study.
Immunohistochemistry for p53 protein
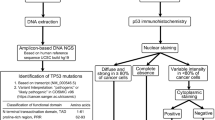
The p53 IHC was performed on 4 μm thick whole slide sections from formalin-fixed paraffin-embedded (FFPE) tumor tissue using anti-p53 (Bp53-11) antibody (Ventana Medical Systems Inc., Tucson, Arizona). The algorithm for p53 IHC interpretation is shown in Fig. 1. A normal pattern of p53 expression (WT, wild-type pattern) was defined as variable staining intensity in < 50% of invasive tumor cells. Abnormal expression of p53 protein in invasive tumor cells were defined as an overexpression pattern (OE, strong and diffuse nuclear staining in at least 80% of tumor cells), null-type pattern (NT, complete absence of expression, with a variable intensity of staining in stromal cells acting as an internal control) [8, 13], a cytoplasmic pattern (CY, strong cytoplasmic staining with absent nuclear staining), or an equivocal pattern (EV, qualitatively and quantitatively greater than that seen in a wild-type pattern but less than that seen in OE) [7]. For discordant cases, the slides were reviewed independently by 3 pathologists (XH, SAA and VLD).
Next-generation sequencing for TP53 mutation
NGS analysis was carried out and reported by one of four CLIA-certified laboratories at the request of the treating physicians. FFPE tumors from 28 patients were subjected to whole-exome sequencing (DNA) for detection of single nucleotide variants (SNVs), insertion and deletion alterations (indels), copy number alterations (CNAs), karyotyping, viruses, and whole transcriptome sequencing (RNA) for gene fusions and variant transcripts (Caris Life Sciences, Inc. Phoenix, AZ). FFPE tumors from 23 patients were subjected to DNA extraction and target enrichment for NGS using the Agilent HaloPlex HS targeted sequencing method (Agilent Technologies, Santa Clara, CA). NGS was also performed using the Illumina MiSeq instrument (Illumina, San Diego, CA [14]). Alterations in 50 genes that are relevant for breast cancer were reported (UAB pathology laboratory). FFPE tumors from 8 patients were subjected to a qualitative NGS that uses targeted high throughput hybridization-based capture technology for detection of substitutions, indels, and CNAs in 324 key cancer-related genes, when using the DNAx extraction method (Foundation Medicine Inc., Cambridge, MA). FFPE tumors from 5 patients were subjected to a NGS targeting 323 genes in DNA coding regions. The genomic alterations include SNPs, indels, rearrangements and other alterations (NeoGenomics Laboratories, Inc. Aliso Viejo, CA). All NGS cover the entire exon with at least a 10 base flanking region of TP53. In this study, pathogenic and likely pathogenic alterations of TP53 gene were considered as carrying a pathogenic mutation; gene alterations with a variant of uncertain significance (VUS) were excluded. For data analysis, TP53 mutation status was subclassified using two-tiers and three-tiers. The two-tier classifier was defined as: TP53 mutated (any detected pathogenic alteration) or TP53 wild-type (no detectable mutation). The three-tier classifier was defined as: missense; truncating including frameshift, nonsense and splice site; or no detectable mutation.
Statistical analysis
A Kruskal–Wallis test was used for group comparisons. A Fisher’s exact test was used to explore the association between two categorical variables. All the tests were two-tailed at a significance level of 0.05.
Results
We identified a total of 97 patients in our database whose tumors underwent NGS per clinical request. Sixty-four patients had available tumor specimens, on which we performed p53 IHC. IHC and sequencing analysis were independently generated. The interpretation of gene mutations and staining patterns was blinded to the independent molecular pathologists and breast pathologists. The detailed TP53 mutations and p53 IHC patterns are shown in Table 1.
Clinicopathological features
The clinicopathological features of the patients stratified by p53 IHC expression and TP53 mutation status (two-tier classification) are shown in Table 2. Of the 64 cases, 35 (55%) cases showed an abnormal p53 IHC expression, and a TP53 mutation was detected in 34 (53%) of these cases. Nottingham histological grade, ER status, and triple-negative breast cancer (TNBC) were significantly associated with an abnormal p53 IHC expression pattern and TP53 mutation. Other analyzed characteristics failed to show significant correlations. The comparison of clinicopathological features between cases with a TP53 missense mutation and those with a truncating mutation are shown in Table 3. A truncating mutation is positively associated with PR negativity and TNBC (p = 0.0366, 0.0135, respectively).
p53 IHC patterns
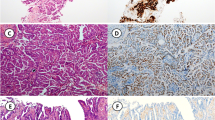
Among the entire cohort of 64 tumors with p53 IHC reactivity, 27% demonstrated an overexpression pattern (17), 22% a null pattern (14), 6% an equivocal pattern (4), and 45% a wild-type pattern (29). All the 17 tumors with a p53 overexpression pattern exhibited unequivocally strong and diffuse nuclear staining in > 80% of the tumor cells. All the 29 tumors classified as p53 wild-type pattern exhibited weak to moderate nuclear staining in < 50% of the tumor cells. There were four tumors which exhibited moderate to strong nuclear staining in > 50% of the tumor cells but did not meet 80% threshold for overexpression. Thus, these four tumors were classified as having an equivocal pattern. None of the tumors exhibited a cytoplasmic pattern. Examples are shown in Fig. 2. The comparison of clinicopathological features between p53 IHC overexpression and null patterns is shown in Table 4. The null pattern is positively associated with TNBC and Nottingham histological grade 3 (p = 0.032, 0.045, respectively).
Association between p53 IHC patterns and TP53 mutation status
Among the 34 tumors with TP53 mutations, the overall subclassifications were 56% missense (19), 21% frameshift (7), 21% nonsense (7), and 3% splice site (1). The comparison of p53 IHC patterns with TP53 mutation status is shown in Table 5A. The concordance was analyzed using a three-tier classification where TP53 missense, truncating or no detectable mutation (NDM) was compared to overexpression, null-type, equivocal, and wild-type pattern. The prevalence of the each of the specific IHC patterns was different across the three TP53 mutation subgroups (Table 5B). The p53 overexpression pattern was present in 79% (15/19) of tumors with a missense mutation, 13% (2/15) with a truncating mutation, and 0% (0/30) with NDM (p < 0.0001). The p53 null-type pattern was present in 5% (1/19) of tumors with a missense mutation, 80% (12/15) with a truncating mutation, and 3% (1/30) with NDM (p < 0.0001). Furthermore, the concordance was analyzed using a two-tier classification schema where the presence or absence of a pathogenic TP53 mutation was compared to a wild-type pattern or abnormal expression. Abnormal expression was present in 97% (33/34) of tumors carrying a TP53 mutation (p < 0.0001, Table 6).
p53 IHC equivocal cases and p53 IHC-TP53 mutation status discordant cases
Four tumors were classified as having an equivocal pattern (Fig. 3). A TP53 mutation was detected in three of the four tumors. Two tumors had discordant results (Fig. 4). One tumor exhibited a p53 wild-type pattern and a TP53 M246V mutation was detected. The other tumor exhibited a p53 null-type pattern but a TP53 mutation was not detected.
Tumors with a p53 IHC equivocal pattern. A1-A2, Case #1, p53 IHC exhibits nuclear staining with moderate to strong intensity in > 50% but < 80% tumor cells. A TP53 p.R273H mutation was detected in the tumor. B1-B2, Case #2, p53 IHC exhibits nuclear staining with moderate to strong intensity in > 50% but < 80% tumor cells. A TP53 p.G245D, p.Y234N mutation was detected. C1-C2, Case #3, p53 IHC exhibits nuclear staining with weak intensity in > 50% but < 80% tumor cells. A TP53 p.R342* mutation was detected in the tumor. D1-D2, Case #4, p53 IHC exhibits nuclear staining with moderate intensity in > 50% but < 80% tumor cells. A TP53 mutation was not detected in the tumor. A1-D1, H&E staining (× 100). A2-D2, p53 IHC staining (× 100)
Discordant cases. A1-A2, Case #1, p53 IHC exhibits wild-type pattern as nuclear staining with moderate intensity in < 50% tumor cells. A TP53 M246V mutation was detected in the tumor. B1-B2, Case #2, p53 IHC exhibits null-type pattern. A TP53 mutation was failed to detect in the tumor. A1-B1, H&E staining (× 100). A2-B2, p53 IHC staining (× 100)
Discussion
This study provides evidence that a p53 IHC assay with appropriate interpretation predicts a TP53 mutation in breast carcinoma, and that a specific p53 IHC pattern can predict a certain type of TP53 mutation. Both abnormal p53 IHC expression and a TP53 mutation show positive correlations with unfavorable pathologic features.
TP53 mutations are associated with the basal-like subtype and poor overall survival in BC patients [15, 16]. In our study, 34 of the 64 (53%) cases carry a TP53 mutation, 19 of them (56%) are missense mutations. A TP53 mutation is associated with a high Nottingham histological grade, ER-negativity, and TNBC, in agreement with previous studies.
Although previous studies showed p53 protein overexpression by IHC was also associated with high risk clinicopathologic features [6, 17], the value of p53 IHC as an accurate predictor of TP53 mutation in BC has not heretofore been fully established [6]. The majority of the TP53 mutations leading to abnormal p53 protein accumulation can be detected by IHC. Most of the previous correlative studies evaluated p53 IHC overexpression and interpreted the results semi-quantitatively, but the accuracy was suboptimal [6, 7]. It was proposed that tumors showing absence of detectable p53 protein (null pattern) also should be considered as carrying a possible TP53 mutation [6]. Köbel et al. optimized their p53 IHC assay, which significantly improved sensitivity and specificity in ovarian carcinoma. They defined abnormal p53 IHC as overexpression, completed absence of expression, or cytoplasmic staining [8]. In our study, 14 of 64 (22%) cases showed a p53 IHC null pattern and 13 of these 14 cases (93%) showed a TP53 mutation (p < 0.00001), compared to 1 of 29 (3%) having a p53 wild-type pattern. Our data further supports the contention that complete absence of p53 IHC staining should be considered as an indication of a TP53 mutation in breast carcinoma.
The role of TP53 mutation in cancer development is complicated and two main mechanisms have been implemented. One is inactivation of the tumor suppressive activity of wild-type p53 by a “loss of function” mechanism; the other is acquiring oncogenic activity by a “gain of function” mechanism [2, 3, 7]. Most TP53 mutations occur in the DNA binding domain and are more frequently caused by missense point mutations [3, 18]. Additionally, studies have shown that certain TP53 mutation types are associated with specific p53 IHC patterns with missense mutations typically resulting in accumulation of p53 protein, while frameshift, nonsense and splice site mutations may result in a null pattern [7]. Alsner et al. reported that the clinical outcome for breast cancer patients is significantly different based on different TP53 mutation types [6, 19]. Their studies showed that missense mutations involved in DNA or zinc binding were associated with the worst outcome, while null mutations and missense mutations within structural/conserved domains were associated with similar but significantly worse outcomes compared to patients with wild-type TP53 [6]. However, the correlations between subtypes of p53 IHC patterns and TP53 mutations need further investigation in BC. In our study, 88% (15/17) of the tumors with a p53 IHC overexpression pattern had a missense mutation in TP53. By comparison, 86% (12/24) of the tumors with a p53 IHC null pattern had a truncating mutation (p < 0.00001). We also identified that tumors with a truncating mutation are more frequently associated with PR negativity and TNBC, compared to those with a missense mutation. As we expected, those cases with a p53 IHC null pattern was positively associated with TNBC and/or Nottingham histological grade 3 compared to those with an overexpression pattern. Further, we note that a p53 IHC staining pattern can predict a specific type of TP53 mutation in BC, i.e., a p53 IHC null pattern is more frequently associated with a TP53 truncating mutation and is associated with unfavorable pathologic features.
Among the 64 cases, 2 tumors showed a discordant p53 IHC pattern/TP53 status. The first tumor had a missense M246V mutation, the p53 IHC showed a wild-type pattern (Fig. 4A). The TP53 M246V missense mutation obscured structure stability [20], resulting in reduced protein thermostability and loss of DNA binding ability [2, 21]. The p53 IHC failed to show an overexpression pattern, which we would have expected to see in tumors carrying a missense mutation. One possible explanation for this is mutated p53 protein degradation due to poor or delayed formalin fixation of the tissue [6, 8]. The second tumor was p53 IHC null-type, but the NGS failed to reveal a TP53 mutation (Fig. 4B). We speculate on 3 possible explanations for this discrepancy. Firstly, this could be false null-type p53 IHC in tumor cells, although the internal stromal cells showed wild-type staining. Secondly, this could be related to the tumor cellularity effected by the admixed stromal cells and lymphocytes in the specimen subjected to NGS (false negative of NGS). Lastly, this could be related to our sequencing approach, which may not have been able to detect all the mutations [22]. The NGS approach may not be able to detect loss of an entire gene or its chromosomal location.
Among the 64 cases, 4 tumors showed equivocal p53 IHC staining (Fig. 3). Three of these 4 tumors had detected TP53 mutations. A missense TP53 mutation in p.R273H was detected in cases #1 (Fig. 3A) and missense mutations in p.G245D and p.Y234N were identified in case #2 (Fig. 3B). Both of the cases showed increased p53 IHC nuclear staining more than typically seen with a wild-type pattern (moderate to strong intensity in > 50% but < 80% of the tumor cells), but failed to reach the threshold identified with an overexpression pattern. Again, the possible explanation for this is mutated p53 protein degradation due to poor or delayed formalin fixation of the tissue [6, 8], as previously mentioned. Elsewhere, a nonsense TP53 p.R342* mutation was detected in case #3 (Fig. 3C), the p53 IHC showed weak nuclear staining in > 50% of the tumor cells. This tumor carried a nonsense TP53 mutation with increased p53 IHC. We speculate a possible explanation being the abnormal p53 protein truncated at the tetramerisation domain within the C-terminus (p.R342*) which may be detected by the p53 Bp53-11 antibody (which binds the transcription domain within the Nh2-terminus). Thus, the IHC staining failed to show a null-type pattern. Lastly, case #4 (Fig. 3D) showed moderate intensity in > 50% but < 80% of the tumor cells by p53 IHC staining with no detectable TP53 mutation. This increased p53 IHC staining could be due to overestimation. For cases with equivocal p53 IHC staining, NGS should be utilized, we suggest, to determine the TP53 mutation status.
In summary, using different IHC patterns to link p53 expression with TP53 mutation status have been proposed and optimized in gynecological and colorectal cancers [7]. Three main patterns of p53 IHC expression are overexpression, null, and wild-type. More recently, additional two patterns have been described in few studies. Köbel et al. [8] and Rabban et al. [13] reported a cytoplasmic pattern which was defined as “strong cytoplasmic staining without nuclear overexpression” in gynecological cancers. Tessier-Cloutier et al. recognized and defined a “basal overexpression” pattern as “uniformly strong nuclear staining in at least 80% of basal cells without significant parabasal staining” in vulvar squamous cell carcinoma [23]. In this study, we propose four p53 IHC patterns: overexpression, null, equivocal and wild-type. We report the first correlational study of p53 IHC patterns and TP53 mutation status in the context of pathological features in breast carcinoma.
Conclusion
This study suggests that a p53 IHC assay, with appropriate interpretation, can predict TP53 mutation status in a large subset of breast carcinomas. Further, a p53 null pattern is associated with a truncating mutation in the TP53 gene and signals worse pathologic features, compared to cases with an overexpression pattern. For the cases with an equivocal p53 IHC expression, further validation studies are required. Additionally, future investigations are necessary to evaluate for the potential diagnostic and therapeutic significance of different p53 IHC patterns and different types of TP53 mutations in breast cancer.
Data availability
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
References
Kastenhuber ER, Lowe SW (2017) Putting p53 in context. Cell 170(6):1062–1078
Wang H, Guo M, Wei H, Chen Y (2023) Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct Target Ther 8(1):92
Marvalim C, Datta A, Lee SC (2023) Role of p53 in breast cancer progression: an insight into p53 targeted therapy. Theranostics 13(4):1421–1442
Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M (2007) TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 26(15):2157–2165
Soussi T (2007) p53 alterations in human cancer: more questions than answers. Oncogene 26(15):2145–2156
Alsner J, Jensen V, Kyndi M, Offersen BV, Vu P, Borresen-Dale AL et al (2008) A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol 47(4):600–607
Bellizzi AM (2023) p53 as Exemplar next-generation immunohistochemical marker: a molecularly informed, pattern-based approach, methodological considerations, and pan-cancer diagnostic applications. Appl Immunohistochem Mol Morphol 31(7):507–530
Kobel M, Piskorz AM, Lee S, Lui S, LePage C, Marass F et al (2016) Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res 2(4):247–258
Matsumoto N, Manrai P, Rottmann D, Wu X, Assem H, Hui P et al (2023) Correlative assessment of p53 immunostaining patterns and TP53 mutation status by next-generation sequencing in high-grade endometrial carcinomas. Int J Gynecol Pathol 42(6):567–575
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 134(6):907–922
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ et al (2007) American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 131(1):18–43
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH et al (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. Arch Pathol Lab Med 138(2):241–256
Rabban JT, Garg K, Ladwig NR, Zaloudek CJ, Devine WP (2021) Cytoplasmic pattern p53 immunoexpression in pelvic and endometrial carcinomas with TP53 mutation involving nuclear localization domains: an uncommon but potential diagnostic pitfall with clinical implications. Am J Surg Pathol 45(11):1441–1451
Morlote D, Janowski KM, Siniard RC, Guo RJ, Winokur T, DeFrank G et al (2019) Effects of improved DNA integrity by punch from tissue blocks as compared to pinpoint extraction from unstained slides on next-generation sequencing quality metrics. Am J Clin Pathol 152(1):27–35
Desmedt C, Voet T, Sotiriou C, Campbell PJ (2012) Next-generation sequencing in breast cancer: first take home messages. Curr Opin Oncol 24(6):597–604
Lai H, Ma F, Trapido E, Meng L, Lai S (2004) Spectrum of p53 tumor suppressor gene mutations and breast cancer survival. Breast Cancer Res Treat 83(1):57–66
Li JP, Zhang XM, Zhang Z, Zheng LH, Jindal S, Liu YJ (2019) Association of p53 expression with poor prognosis in patients with triple-negative breast invasive ductal carcinoma. Medicine 98(18):e15449
Ungerleider NA, Rao SG, Shahbandi A, Yee D, Niu T, Frey WD et al (2018) Breast cancer survival predicted by TP53 mutation status differs markedly depending on treatment. Breast Cancer Res 20(1):115
Alsner J, Yilmaz M, Guldberg P, Hansen LL, Overgaard J (2000) Heterogeneity in the clinical phenotype of TP53 mutations in breast cancer patients. Clin Cancer Res 6(10):3923–3931
Gomes AS, Ramos H, Inga A, Sousa E, Saraiva L (2021) Structural and drug targeting insights on mutant p53. Cancers (Basel). 13(13):3344
Li J, Meeks H, Feng BJ, Healey S, Thorne H, Makunin I et al (2016) Targeted massively parallel sequencing of a panel of putative breast cancer susceptibility genes in a large cohort of multiple-case breast and ovarian cancer families. J Med Genet 53(1):34–42
Cirulli ET, Singh A, Shianna KV, Ge D, Smith JP, Maia JM et al (2010) Screening the human exome: a comparison of whole genome and whole transcriptome sequencing. Genome Biol 11(5):R57
Tessier-Cloutier B, Kortekaas KE, Thompson E, Pors J, Chen J, Ho J et al (2020) Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod Pathol 33(8):1595–1605
Acknowledgements
We thank Sandra S. Moultrie for her excellent assistance with immunohistochemistry.
Funding
The study was supported in part by the University of Alabama Department of Pathology clinical research awards to X.H. and faculty startup funds to X.H.
Author information
Authors and Affiliations
Contributions
SW and XH contributed to study design. SAA, BBB, SH, VLD and XH contributed to data collection. SAA, SH, GPS, and XH contributed to manuscript editing. SH, GPS, SW and XH contributed to manuscript reviewing. SH and XH contributed to statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
No authors have any conflicts of interest to disclose.
Ethical approval
This study protocol was reviewed and approved by the University of Alabama at Birmingham Institutional Review Board (IRB)-approved protocol (IRB-300006547).
Consent to participate and publish
Since this was a retrospective research on archival material, written informed patient consent was not required by decision of the University of Alabama at Birmingham Institutional Review Board (IRB).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anderson, S.A., Bartow, B.B., Harada, S. et al. p53 protein expression patterns associated with TP53 mutations in breast carcinoma. Breast Cancer Res Treat 207, 213–222 (2024). https://doi.org/10.1007/s10549-024-07357-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07357-z