Abstract
Purpose
Central lumpectomy (CL) is a breast-conserving surgical (BCS) technique that involves excision of the nipple-areolar complex with breast tumor in centrally located breast cancers. We aimed to investigate the long-term clinical outcomes of CL in comparison with conventional BCS (cBCS).
Methods
Patient records who underwent BCS with clear resection margins for invasive breast cancer between 2004 and 2018 were retrospectively reviewed. Of the total 6,533 patients, 106 (1.6%) underwent CL. Median follow-up duration was 73.4 months. 1:3 propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) were used to minimize selection bias.
Results
The CL group showed a significantly higher ipsilateral breast tumor recurrence (IBTR) rate than the cBCS group (10-year IBTR rate: 5.8% vs. 3.1%, p = 0.004), even after adjusting for other variables (hazard ratio (HR), 2.65; 95% confidence interval (CI), 1.07–6.60, p = 0.048). However, there were no significant differences observed in regional recurrence, distant metastasis, or overall survival rates between the two groups. Both PSM and IPTW analyses showed significantly higher IBTR in the CL group (PSM HR, 3.27; 95% CI, 0.94–11.36; p = 0.048 and IPTW HR, 4.66; 95%CI, 1.85–11.77; p < 0.001). Lastly, when analyzing 2,213 patients whose tumors were located within 3 cm of the nipple, the CL group showed a significantly higher IBTR than the cBCS group before and after PSM.
Conclusion
CL was associated with a higher rate of IBTR compared to cBCS, while other survival outcomes were comparable. For centrally located tumors, CL may be considered for patients preferring breast preservation. However, higher risk for IBTR should be informed and careful surveillance may be necessary during the early post-operative follow-up periods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast-conserving surgery (BCS) is a well-established and preferred treatment for the majority of patients with early breast cancer. Combined with radiation, BCS can offer patients better cosmetic satisfaction and equivalent oncologic outcomes compared to mastectomy [1]. In contrast, mastectomy is preferred for centrally located breast cancer (CLBC) due to concern for oncologic safety regarding tumor involvement of the nipple-areolar complex (NAC) and failure to achieve satisfactory cosmetic outcomes due to resection of the NAC. Further, a previous study has shown that BCS with preservation of the NAC is feasible only for strictly selected patients [2].
Central lumpectomy (CL) is a BCS technique that excises NAC with breast tumors. Considering that breast conservation is associated with improved patient satisfaction compared to mastectomy, CL is a reasonable surgical option for patients who strongly wish to conserve their breasts [3]. Several studies have reported superior satisfaction and quality of life following BCS compared to mastectomy and/or reconstruction [3, 4]. It is also known that centrally located breast tumors are not an absolute contraindication for BCS [5].
Several studies have investigated the survival of patients undergoing CL [6, 7]. A recent study using the SEER database reported a comparable overall survival rate of BCS for CLBC compared to mastectomy [7]. Information regarding the rate of local recurrence following CL, however, is limited. Another SEER study compared the oncologic safety of BCS for CLBC with that of BCS for non-CLBC and showed comparable oncologic outcomes in terms of 5-year local recurrence-free survival. The analysis, however, was confined to stage I and II breast cancers [6]. Recently, neoadjuvant chemotherapy has led to an increased rate of BCS [8], suggesting that more advanced-stage patients are eligible for CL. Consequently, the oncologic outcomes of CL warrant reassessment to reflect the recent shift toward increased adoption of BCS.
This study investigates the long-term clinical outcomes of CL compared to conventional BCS by PSM to reduce confounding and minimize selection bias. We also evaluate tumor-to-nipple distance to identify confounders based on tumor location.
Methods
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H2209-011–1355). The Declaration of Helsinki and good clinical practice guidelines were followed, and the need for informed consent was waived.
Study design
We retrospectively obtained baseline clinicopathological data of patients who underwent BCS for invasive breast cancer between January 2004 and December 2018 from the database of the Seoul National University Hospital Breast Care Center. We excluded patients with stage IV breast cancer, bilateral breast cancer, male breast cancer, recurrent breast cancer, or synchronous or metachronous cancer in other organs. Bilateral breast cancer was excluded due to higher risk of recurrence and likelihood of carrying BRCA mutations. As the involved resection margin was strongly associated with IBTR [9], our study only included patients with clear margins, defined as “no ink on tumor.” The initial breast cancer was clinically or pathologically staged according to the 7th American Joint Committee on Cancer staging criteria. The distance from the nipple was collected from preoperative magnetic resonance imaging and breast sonography reports. Hormone receptor status, including estrogen and/or progesterone receptors, was defined as positive when dyed > 1% on immunohistochemistry. Human epidermal growth factor receptor type 2 (HER2) status was assessed using anti-HER2 antibodies and/or fluorescence in situ hybridization. A Ki-67 level with positivity of < 10% was defined as low according to a previous study conducted in our institution [10].
Surgical methods for breast conservation
CL was considered when the tumor was located close to the nipple on breast radiologic exams including breast sonography and breast MRI and when nipple excision was deemed inevitable. All patients with suspected invasion to the nipple were given the choice of whether to receive CL or mastectomy and shared decision-making was made by providing detailed description of surgical procedures. Nonetheless, the final decision leading up to CL primarily relied on the surgeon’s discretion due to cultural preference for passive acceptance of the doctor’s care. Most patients who underwent CL were approached through an elliptical incision or circumareolar incision (supplementary fig.S1). Besides, there was no patient who voluntarily selected CL although nipple conservation was possible. Notably, two patients whose tumors were located far from the nipple but underwent NAC resection for the purpose of oncoplastic surgery were excluded from the current study. The conventional BCS group only included patients who underwent oncoplastic surgery with NAC preserved. The choice of surgical methods did not affect the subsequent radiotherapy treatment regimen or fractionation schedules.
Pathologic process for resection margin assessment
Following lumpectomy, all six margins of the excised mass were marked with distinct ink colors. Subsequently, the specimen was sliced at 5-mm intervals. Final margin was considered negative/clear when no tumor cell touched the ink on any of the six surfaces of the lumpectomy specimen.
Recurrence and recurrence-free survival
Ipsilateral breast tumor recurrence (IBTR), the primary endpoint of the current study, was defined as the first recurrence in any quadrant of the ipsilateral breast. Regional recurrence was defined as recurrence in the ipsilateral regional lymph nodes, including the axillary, supraclavicular, infraclavicular, and internal mammary nodes. Recurrence-free survival was defined as the time interval between the date of surgery and pathologic or radiologic confirmation of recurrence. Regarding competing risks, other types of recurrence, including regional recurrence or distant metastasis prior to IBTR, were treated as censored events when calculating the IBTR-free survival rate. Overall survival (OS) was defined as the interval from surgery to death or last follow-up.
Statistical analysis
Categorical variables and continuous variables were compared using Pearson’s χ2 test and one-way analysis of variance, respectively. The survival curves were derived using the Kaplan–Meier method, and the difference was analyzed using the log-rank test. The Cox proportional hazard regression model was used to estimate the adjusted hazard ratio and adjust for variables associated with the recurrence rate. Using Cox regression analysis, we adjusted for clinicopathological variables affecting IBTR, such as age at operation, tumor stage, histologic grade, lymphovascular invasion, hormone receptor status, HER2 status, Ki-67 level, and adjuvant treatment. Further, to minimize potential selection bias between the two groups, we conducted 1:3 PSM, including age at operation, year of surgery, tumor stage, histologic grade, lymphovascular invasion, hormone receptor status, HER2 status, Ki-67 level, and administration of neoadjuvant and adjuvant treatments. Estimated propensity score was further used to conduct inverse probability of treatment weighting (IPTW) analysis. Statistical significance was set at p < 0.05. All analyses were performed using SPSS (version 27.0; SPSS, Inc., IBM, Armonk, USA), and figures were plotted using GraphPad Prism™ (version 9.0; GraphPad Software, San Diego, CA, USA). PSM was conducted with “MatchIt” R package (version 3.6.3) [11].
Results
Patient demographics and characteristics
A total of 6,533 patients were identified who underwent BCS and met our inclusion criteria. For all patients, mean age at operation was 50.0 ± 9.8 years (range, 19.0–88.0). Most patients had hormone receptor-positive (73.3%) and/or HER2-negative (83.5%) tumors. Neoadjuvant chemotherapy was administered to 1,243 patients (19.0%) and 6,187 patients (94.7%) received adjuvant radiotherapy. The clinicopathological characteristics of the patients are summarized in Table 1.
Overall, 106 (1.6%) patients underwent CL, with the remaining 6,427 (98.4%) undergoing conventional BCS. Compared to the conventional BCS group, patients in the CL group underwent surgery more recently and showed more lymphovascular invasion, hormone receptor positivity, and HER2 positivity. Neoadjuvant chemotherapy and radiotherapy were also administered more frequently in the conventional BCS group compared to the CL group (Table 1).
Impact of surgical technique on survival outcomes
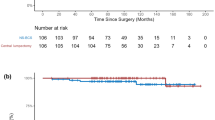
During the median follow-up period of 73.4 months, six (5.7%) and 134 (2.1%) IBTR events were noted in the CL and conventional BCS groups, respectively. The CL group had significantly higher IBTR than the conventional BCS group (10-year IBTR: 5.8% vs. 3.1%; hazard ratio [HR], 3.09; 95% confidence interval [CI], 1.36–7.01; Log-rank p = 0.004) (Fig. 1A). In contrast, RR-free survival, distant metastasis (DM)-free survival (DMFS), and OS rates were not significantly different between the two groups (Supplementary Fig. S2).
Kaplan–Meier curves for ipsilateral breast tumor recurrence-free survival comparing the surgical methods. The Kaplan–Meier curves show the differences in IBTR between the two groups before (A) and after (B) 1:3 propensity score matching. P-values were calculated using the log-rank test and hazard ratios were calculated using the Cox regression test. BCS breast-conserving surgery, CI confidence interval, IBTR ipsilateral breast tumor recurrence
After adjusting for clinicopathological variables, surgical strategy for lumpectomy remained a significant prognostic variable associated with IBTR (HR, 2.65; 95%CI, 1.07–6.60, p = 0.048) (Table 2). In addition, younger age, higher histologic grade, lymphovascular invasion, no adjuvant chemotherapy, and no radiotherapy were associated with higher IBTR (p < 0.001).
Additionally, we conducted PSM yielding 99 and 297 patients in the CL and conventional BCS groups, respectively (Table 3). After matching, IBTR events were observed in five cases each in both the CL (5.1%) and conventional BCS (1.7%) groups. Similar to the comparison of the original unmatched cohorts, the CL group was associated with a significantly higher IBTR than the conventional BCS group on log-rank test (HR, 3.27; 95%CI, 0.94–11.36, log-rank p = 0.048) (Fig. 1B), while the recurrence-free and OS rates were also comparable between the two groups (Supplementary Fig. S3). Lastly, we conducted IPTW to further reduce selection bias by giving more weights to less common, but potentially more informative population. The effective sample size was the same as the original cohort, but weighted survival analysis still showed a significantly higher IBTR for the CL group compared to the conventional BCS group (HR, 4.66; 95%CI, 1.85–11.77; p < 0.001) (Supplementary Fig. S4).
Survival analysis for tumors located near the NAC
Because tumors in the CL group were located more centrally near the nipple, the distance between the NAC and tumors was significantly shorter than that in the conventional BCS group (p < 0.001, Fig. 2). To determine the impact of tumor-nipple distance on survival outcomes, we selected tumors located within 3 cm from the nipple on preoperative MRI or breast sonography, yielding 100 and 2,113 patients in the CL and conventional BCS groups, respectively. After this selection, the CL group remained associated with higher IBTR than the conventional BCS group (HR, 2.44; 95%CI, 1.05–5.64, log-rank p = 0.032) (Fig. 3A). Furthermore, we conducted a 1:3 PSM for these patients (Supplementary table S1). Among the 96 and 288 patients in each group, the CL group continued to show higher IBTR than the conventional BCS group (HR, 4.04; 95%CI, 1.08–15.11, log-rank p = 0.025) (Fig. 3B).
Location of the tumors relative to the nipple following propensity score matching. Following 1:3 propensity score matching, the tumor locations of 396 patients relative to the nipple are shown. Tumors associated with IBTR during follow-up are shown as filled circles (A). The distance from the nipple was significantly shorter in the CL group than in the conventional BCS group (B). BCS breast-conserving surgery, SD standard deviation, IBTR ipsilateral breast tumor recurrence
Kaplan–Meier curves for patients with tumor location within 3 cm from the nipple. Kaplan–Meier curves show the differences in IBTR between the two groups before (A) and after (B) 1:3 propensity score matching. P-values were calculated using the log-rank test and hazard ratios were calculated using the Cox regression test. IBTR ipsilateral breast tumor recurrence, BCS breast-conserving surgery, CI confidence interval
Recurrence pattern of IBTR after surgery
The pattern of IBTR for those who underwent conventional BCS showed a double-peaked pattern, with the first peak at year 2 and the second peak between years 8 and 9 after surgery, which was similar to our previous report on locoregional recurrence patterns [12]. On contrast, the pattern of IBTR for the CL group showed significantly higher incidence until the first 6 years of surgery, but dramatically decreased thereafter. (Supplementary Fig. S5).
Discussion
In the current study, we found CL to be associated with significantly higher IBTR compared to conventional BCS before and after PSM. The results were consistent for tumors confined to 3 cm from the nipple. In contrast, there was no significant difference in regional and distant recurrence, as well as OS, based on the surgical method.
Mastectomy is frequently favored for CLBCs due to concern for oncologic safety and apprehensions regarding unsatisfactory cosmetic outcomes following CL [13]. Recent studies, however, have reported the safety of BCS for CLBC and introduced several surgical techniques [6, 7, 14, 15]. In addition, with the development of NAC reconstruction methods, the disadvantages of CL can be reduced [16]. Considering that BCS can achieve higher cosmetic satisfaction and quality of life than total mastectomy, the oncologic safety of CL warranted studying [3, 4]. Few studies, however, have investigated locoregional recurrence following CL. Our findings suggest that although CL can be regarded as oncologically safe, post-operative surveillance of IBTR should be performed.
Several reports have studied the survival of patients undergoing BCS for CLBC. Simmons et al. retrospectively analyzed 99 patients and reported that the local or distant recurrence rate of central breast cancer did not differ significantly between lumpectomy and mastectomy at a median follow-up of 33 months (local recurrence, 6.3% vs. 3.5%; distant recurrence, 3.1% vs. 3.0; both p > 0.99)[17]. Another retrospective study which analyzed 333 patients compared BCS with and without nipple resection and showed comparable breast-free survival rates between the two groups [18]. These findings were supported by two SEER data studies [7, 19]. Liu et al. analyzed 8,702 patients (3,870 and 4,832 patients in the BCS (including CL) and non-BCS group, respectively) with CLBC and showed that the BCS group had a higher breast cancer-specific survival rate (p < 0.001) and OS rate (p < 0.001) than the non-BCS group. Fitzal et al. reviewed 1,485 patients (105, 1312, and 68 patients with CL for CLBC, mastectomy for CLBC, and conventional BCS for non-CLBC, respectively) and found no difference in OS (p = 0.348) and recurrence-free survival (p = 0.649) between CL and mastectomy for CLBC at a median follow-up of 35.3 months. Further, they failed to show a difference in local recurrence (p = 226) between the CL and conventional BCS groups. Unlike previous studies, we found a higher local recurrence rate following CL. This may be because previous studies only included patients with stage I and II breast cancer and excluded patients who underwent neoadjuvant chemotherapy. Our study included patients with stage III disease and those who received neoadjuvant chemotherapy. Additionally, adjustment for other variables with Cox regression analysis, PSM, and IPTW, along with a longer follow-up period, supports our results.
CL is mainly performed for subareolar tumors. Breast cancers originating from the major lactiferous ducts are reported to have different clinical, histopathological, and mammographic presentations compared to tumors originating from the terminal ductal and lobular units (TDLU) [20]. In the major ducts, cancer cells distend and distort normal ductal structures and often form new duct-like structures with massive tumor burdens. Tabar et al. reported that the 24-year cumulative survival of invasive breast cancer patients with both TDLUs and the main ductal component involvement was poorer than patients with tumors originating from the TDLU portion only (RR, 9.04; 95%CI, 4.78–17.08) [21]. Anatomically, the major lactiferous ducts converge into the nipple, and there is a higher risk for tumors close to the nipple to invade the major ducts. These findings suggest that tumors in the central portion of the breast may behave differently compared to tumors originating from other parts of the breast, resulting in different survival outcomes.
Furthermore, the observation that recurrent tumors in the CL group of the current study were not localized solely around the nipple, and that the distance between the recurrent tumor and the nipple was not significantly different between the two groups, provides further support for this theory. (Supplementary Fig. S6).
Breast tumor location may be an independent prognostic factor for survival outcomes [21, 22]. According to published studies, the prognosis of breast cancer varies depending on the tumor location in the breast, with the best prognosis for tumors in the upper outer quadrant. However, central breast cancers have limited data. Ji et al. reported greater disease severity and poorer survival of patients with tumors in the central and nipple portions [23]. They showed that tumors located in the central and nipple portion were associated with older age, larger tumor size, advanced tumor stage, and axillary node metastasis, which resulted in poorer breast cancer-specific survival (p = 0.005) and OS (p < 0.0001). Accordingly, we sub-analyzed tumors located 3 cm from the NAC in an effort to nullify the effect of distance to the nipple on survival outcome. The results showed patients undergoing CL to have a higher IBTR despite PSM.
The current study had several limitations. First, this retrospective study from a single institution may be associated with selection bias. Other variables affecting IBTR events such as the administration of radiation boost and multifocal lesions were not investigated. However, most of the patients underwent radiotherapy and achieved a clear resection margin in pathology reports, which are the strongest predictors of IBTR. Small number of patients in the CL group compared to majority of patients in the conventional BCS group is a mismatched comparison and requires caution in interpretation, notwithstanding our sensitivity analyses including multivariate analysis and PSM resulted in consistent result. Another limitation is subjectivity in the process leading to CL. CL was considered when the tumor was located close to the nipple and when nipple excision was deemed inevitable. Retrospective analysis on the location of the tumors showed that most of the tumors in the CL group were located within 3 cm from the nipple. However, there was no strict criteria for “close” distance when making the choice for surgical technique, and it heavily relied on the surgeon’s discretion and size of the breast. Due to these limitations, our current results warrant careful interpretation and multi-institutional investigations with a larger number of heterogeneous patients are needed to strongly support our findings. Finally, despite our PSM effort, we could not completely nullify the effect of the distance from the nipple, and the distance remained shorter in the CL group (Supplementary Fig. S7). Therefore, our study emphasizes on the comparison of surgical technique, rather than examining tumors only located under or close to the NAC area.
The absolute difference in IBTR between the CL group and the conventional BCS group was small, although it was statistically significant. Therefore, in the absence of any other differences in outcome, it would not be justified to perform mastectomy, alter RT dose or extent, or administer additional systemic therapy for patients who are eligible to receive CL.
In conclusion, patients who undergo CL have comparable DMFS and OS, but higher IBTR than those undergoing conventional BCS. For patients with CLBC, higher risk for IBTR associated with breast conservation should be informed before performing CL, and careful surveillance may be necessary during the early post-operative follow-up periods.
Data availability
Ji-Jung Jung and Jong-Ho Cheun had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The datasets analyzed during the current study are not publicly available due to institutional regulations, but are available from the corresponding author on reasonable request.
References
van Maaren MC, de Munck L, de Bock GH et al (2016) 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 17(8):1158–1170. https://doi.org/10.1016/S1470-2045(16)30067-5
Dale PS, Giuliano AE (1996) Nipple-areolar preservation during breast-conserving therapy for subareolar breast carcinomas. Arch Surg 131(4):430–433. https://doi.org/10.1001/archsurg.1996.01430160088019
Atisha DM, Rushing CN, Samsa GP et al (2015) A national snapshot of satisfaction with breast cancer procedures. Ann Surg Oncol 22(2):361–369. https://doi.org/10.1245/s10434-014-4246-9
Flanagan MR, Zabor EC, Romanoff A et al (2019) A comparison of patient-reported outcomes after breast-conserving surgery and mastectomy with implant breast reconstruction. Ann Surg Oncol 26(10):3133–3140. https://doi.org/10.1245/s10434-019-07548-9
Torosian M. Breast Cancer: A Guide to Detection and Multidisciplinary Therapy (Current Clinical Oncology). NJ: Totowa; 2002:2002nd ed. Humana Press. https://doi.org/10.1007/978-1-59259-161-9_5.
Shen YF, Huang J, Zhou WB et al (2022) Breast-conserving in centrally located breast cancer patients confirmed safe by SEER based study. Gland Surg 11(1):226–235. https://doi.org/10.21037/gs-21-914
Liu J, Zheng X, Lin S, Han H, Xu C (2022) Breast conserving therapy for central breast cancer in the United States. BMC Surg 22(1):31. https://doi.org/10.1186/s12893-022-01488-0
Petruolo O, Sevilimedu MG, Le T, Morrow M, Barrio AV (2021) Often does modern how often does modern neoadjuvant chemotherapy downstage patients to breast-conserving surgery? Ann Surg Oncol 28(1):287–294. https://doi.org/10.1245/s10434-020-08593-5
Houssami N, Macaskill P, Marinovich ML, Morrow M (2014) The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol 21(3):717–730. https://doi.org/10.1245/s10434-014-3480-5
Jung SY, Han W, Lee JW et al (2009) Ki-67 expression gives additional prognostic information on St. Gallen 2007 and adjuvant! online risk categories in early breast cancer. Ann Surg Oncol. 16(5):1112–1121. https://doi.org/10.1245/s10434-009-0334-7
Ho DE, Imai K, King G, Stuart EA (2011) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 42:8. https://doi.org/10.18637/jss.v042.i08
Cheun JH, Kim HK, Moon HG et al (2023) Locoregional recurrence patterns in patients with different molecular subtypes of breast cancer. JAMA Surg 158(8):841–852. https://doi.org/10.1001/jamasurg.2023.2150
Kroman N, Holtveg H, Wohlfahrt J et al (2004) Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer 100(4):688–693. https://doi.org/10.1002/cncr.20022
Kijima Y, Yoshinaka H, Shinden Y et al (2014) Oncoplastic breast surgery for centrally located breast cancer: a case series. Gland Surg 3(1):62–73. https://doi.org/10.3978/j.issn.2227-684X.2013.11.01
Eggemann H, Ignatov A, Elling D et al (2013) Efficacy and patient satisfaction of breast conserving therapy for central breast cancer by the B technique. Ann Surg Oncol 20(11):3438–3445. https://doi.org/10.1245/s10434-013-3030-6
Kristoffersen CM, Seland H, Hansson E (2017) A systematic review of risks and benefits with nipple-areola-reconstruction. J Plast Surg Hand Surg 51(5):287–295. https://doi.org/10.1080/2000656X.2016.1251935
Simmons RM, Brennan MB, Christos P, Sckolnick M, Osborne M (2001) Recurrence rates in patients with central or retroareolar breast cancers treated with mastectomy or lumpectomy. Am J Surg 182(4):325–329. https://doi.org/10.1016/S0002-9610(01)00721-8
Horiguchi J, Koibuchi Y, Iijima K et al (2005) Local control by breast-conserving surgery with nipple resection. Anticancer Res 25(4):2957–2959
Fitzal F, Mittlboeck M, Trischler H et al (2008) Breast-conserving therapy for centrally located breast cancer. Ann Surg 247(3):470–476. https://doi.org/10.1097/SLA.0b013e31815b6991
Tabár L, Dean PB, Lee Tucker F et al (2022) A new approach to breast cancer terminology based on the anatomic site of tumour origin: The importance of radiologic imaging biomarkers. Eur J Radiol 149:110189. https://doi.org/10.1016/j.ejrad.2022.110189
Tabár L, Dean PB, Lee Tucker F et al (2022) Breast cancers originating from the major lactiferous ducts and the process of neoductgenesis: Ductal adenocarcinoma of the Breast. DAB Eur J Radiol 153:110363. https://doi.org/10.1016/j.ejrad.2022.110363
Sohn VY, Arthurs ZM, Sebesta JA, Brown TA (2008) Primary tumor location impacts breast cancer survival. Am J Surg 195(5):641–644. https://doi.org/10.1016/j.amjsurg.2007.12.039
Ji F, Xiao WK, Yang CQ et al (2019) Tumor location of the central and nipple portion is associated with impaired survival for women with breast cancer. Cancer Manag Res 11:2915–2925. https://doi.org/10.2147/CMAR.S186205
Funding
Open Access funding enabled and organized by Seoul National University. This research was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2022R1A2C2007561 to W.H.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ji-Jung Jung and Jong-Ho Cheun. The first draft of the manuscript was written by Ji-Jung Jung and Jong-Ho Cheun and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Han-Byoel Lee and Wonshik Han report being a member on the board of directors of and holding stock and ownership interests at DCGen, Co., Ltd., not relevant to this study. Other authors declare no competing interests.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki or comparable ethical standards. Approval was granted by the ethics committee or institutional review board at the participating sites.
Consent to participate
Requirement for informed consent was waived for all patients.
Consent to publish
All authors have read the paper and consent to its publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jung, JJ., Cheun, JH., Kim, HK. et al. Comparison of long-term oncologic outcomes of central lumpectomy and conventional breast-conserving surgery for invasive breast cancer: propensity score matching analysis. Breast Cancer Res Treat 205, 465–474 (2024). https://doi.org/10.1007/s10549-024-07297-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-024-07297-8