Abstract
Purpose
Aromatase inhibitor (AI) therapy reduces risk of recurrence and death for postmenopausal women with breast cancer (BC); however, AI-induced arthralgia (AIIA) can lead to discontinuation of treatment. Curcumin, a bioactive polyphenolic substance, may help ameliorate inflammation-related conditions including osteoarthritis and pain.
Methods
We conducted a multisite randomized placebo-controlled, double-blind pilot trial (Alliance A22_Pilot9) to evaluate the effects of nanoemulsion curcumin (NEC, 200 mg/day) in postmenopausal women experiencing AIIA for ≥ 3 months. The primary objective was to determine the feasibility of using Functional Assessment of Cancer Treatment-Endocrine Symptoms (FACT-ES) to detect changes from 0 (T0) to 3 months (T3) of NEC treatment in AI-induced symptoms and well-being; secondary objectives included evaluation of changes in Disabilities of the Shoulder, Arm, and Hand (DASH), Brief Pain Inventory-short form (BPI-SF), grip strength, and biomarkers at T0 and T3.
Results
Forty-two patients were randomized to NEC or placebo; 34 women completed the 3-month study. Patient-reported outcome measures (PROMs: FACT-ES, DASH, BPI-SF) and biospecimens were collected at T0-T3 in > 80% of participants. Adherence was ≥ 90% for both arms. PROMs and grip strength did not differ significantly by treatment arm. Plasma curcumin was detected only in NEC arm participants. Serum estradiol and estrone levels were below detection or low on study agent. Gastrointestinal adverse effects were commonly reported in both arms.
Conclusion
NEC versus placebo in a multisite randomized trial is feasible and well-tolerated. Additional studies with larger sample size are needed to further evaluate the efficacy and safety of NEC in treatment of AIIA.
ClinicalTrials.gov Identifier: NCT03865992, first posted March 7, 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatase inhibitor (AI) therapy reduces risk of recurrence and death from estrogen receptor (ER)- and/or progesterone receptor (PR)-positive breast cancer (BC). By preventing peripheral conversion of tissues such as fat to estrogen in postmenopausal women, AIs cause profound estrogen deprivation. Recent studies also indicate benefits of prolonged therapy with these drugs for 7–10 years in patients with higher risk BC[1,2,3].
Despite the efficacy of AI therapy for postmenopausal ER-positive BC, side effects may prove limiting. Musculoskeletal symptoms of joint pain and stiffness affect up to 25–80% of women taking AIs[4, 5]. Detrimental effects of AI-induced arthropathy (AIIA) include diminished quality of life, reduced adherence, and even discontinuation of treatment[6]. While nonsteroidal anti-inflammatory drugs (NSAIDs) may help relieve symptoms of joint arthropathy, such medications are not uniformly well-tolerated due to gastrointestinal and renal toxicity. Two recent randomized clinical trials showed reductions in AIIA with (1) duloxetine versus placebo at 12 weeks[7] and (2) acupuncture versus sham/wait list control at 6 weeks[8]. Despite the reported reduction in AI-induced joint symptoms, the side effects, possible drug interactions (duloxetine), high cost, and limited clinical acupuncture access may hinder the broad uptake of such interventions. Natural products such as omega-3 fatty acids have also been evaluated for treatment of AIIA[9], with post hoc analyses showing improved symptoms in obese versus non-obese women[10].
A naturally occurring polyphenol derived from turmeric root (Curcuma longa), curcumin is a widely used spice in Asian and Middle Eastern food. Turmeric also has a long history of medicinal use in China and India. As the bioactive ingredient of turmeric, curcumin is highly investigated as a natural product with potent anti-inflammatory and antioxidant properties[11, 12].
Several clinical trials support a potential role for curcumin in management of joint pain and stiffness. A registry study of Meriva®, a phosphatidylcholine complex of curcumin, showed improved walking distance in osteoarthritis patients with mild to moderate knee pain inadequately controlled by NSAIDs[13]. In a recent meta-analysis of randomized clinical trials of curcumin/turmeric for osteoarthritis or rheumatoid arthritis symptoms, 7 studies reported reduced pain and arthritis symptoms with curcumin/turmeric[14]. Clinical trials of curcumin have also indicated improvement in other conditions associated with AI therapy, such as depression/depressive symptoms[15,16,17], cognitive deficits[18, 19], and dyslipidemia[20].
The limited oral bioavailability (< 2%) and rapid hepatic metabolism of curcumin have posed obstacles to its clinical use. Development of novel nutraceutical delivery systems to enhance absorption and bioavailability of curcumin has included nanoemulsifying drug delivery systems using an isotropic mixture of surfactant and drug[21]. The surfactant-induced membrane fluidity and permeability changes improve drug bioavailability by enhancing solubilization, absorption, and protection against enzymatic hydrolysis[22, 23]. As an ideal vehicle for hydrophobic compounds such as curcumin, we developed a novel nanoemulsifying curcumin (NEC) that delivers much higher plasma levels of curcumin and its major metabolite curcumin-O-glucuronide in mice compared to suspension curcumin[23].
Taken together, these data led us to hypothesize that curcumin administered as a nanoemulsion might alleviate symptoms resulting from AI therapy and enable completion of the recommended treatment. We conducted a randomized double-blind pilot trial of NEC vs placebo in postmenopausal women with AIIA ≥ 3 months to determine feasibility of detecting changes in patient-reported outcome measures (PROMs), grip strength, plasma curcumin levels, and estrogen biomarkers following curcumin exposure.
Methods
Study oversight
This was a multi-institutional randomized, double-blind, placebo-controlled pilot study of NEC approved by the institutional review boards of City of Hope and The Ohio State University. The trial was conducted in accordance with institutional guidelines. Enrollment opened 4/2019 and closed 1/2021. Study participants were informed of the investigational nature of the study and provided written informed consent.
Patient eligibility criteria
Eligible participants were postmenopausal women with stage I-IIIa ER- and/or PR-positive BC who reported at least 3 months or worsening of joint pain resulting from ongoing adjuvant AI therapy. Eligibility requirements included AIIA with score of ≥ 4, based on ≥ 3[24, 25] to ≥ 5[9] thresholds in prior AIIA trials, for the worst pain question of BPI-SF (0 for ‘no pain’ to 10 for ‘pain as bad as can imagine’). Exclusion criteria included: other prior invasive malignancy ≤ 5 years except treated non-melanoma skin cancer; history of anemia; bone fracture or surgery of affected joints < 180 days; medical therapy, alternative therapy, or physical therapy for joint symptoms ≤ 30 days or interarticular steroids ≤ 90 days; use of analgesics other than NSAIDs, acetaminophen < 14 days of study entry; use of medications that induce/inhibit CYP3A4; use of herbal/dietary supplements containing curcumin/curcuminoids ≤ 3 months of study entry; known sensitivity or allergy to turmeric or curry.
Study intervention
Following signed informed consent, study participants were scheduled for the baseline visit (month 0) and randomized 1:1 to NEC or placebo. Only the pharmacist who dispensed the study agent was aware of randomization assignment. Women were advised to take 1 capsule twice daily for 3 months to provide either 200 mg/d of curcumin or placebo. Study capsules for NEC (lot #6103498) and matching placebo (lot #6103497) were manufactured by Lonza, Greenwood, SC. Each active capsule contained approximately 100 mg curcuminoids (Curcumin C3-complex®, Sabinsa, East Windsor, NH). Placebo capsules contained the same excipients, with food-safe dyes mixed to match the color and appearance of NEC-containing capsules.
Participants were instructed to complete daily logs of study capsule doses taken, use of analgesics, change in medications, and adverse events/symptoms. Daily logs and pill counts were reviewed monthly. Anthropometric measurements were taken at months 0 (T0) and 3 (T3). Grip strength was quantitated by hand dynamometer (JAMAR®, JLW instruments, Chicago, IL; Trailite Digital Hand dynamometer, Zhongshan, China) at T0 and T3; two trials were taken with each hand via squeezing the dynamometer for 2–3 s and releasing with a period of one-minute rest between trials. Blood samples were collected at T0 and T3 for complete blood count (CBC) and alanine aminotransferase (ALT) by institutional clinical laboratories. Plasma/serum aliquots were stored at − 80 °C for curcumin, estrone (E1) and estradiol (E2) levels for future batch analysis.
PROMs
We used three tools to assess AIIA: (1) Functional Assessment of Cancer Therapy-Endocrine Symptoms (FACT-ES) measures physical, social and family, emotional, and functional well-being and endocrine symptoms with five response levels scored 0–4[26]; (2) Disabilities of the Arm, Shoulder and Hand (DASH) measures upper extremity physical function and joint symptoms, with 30 items scored 1–5 for health status over the past week regarding the degree of difficulty performing different physical activities due to arm, shoulder, and hand problems, severity of symptoms of pain, activity-related pain, tingling, weakness, and stiffness, and impact on social functioning, work, sleep, and self-image[27]; and (3) Brief Pain Inventory Short Form (BPI-SF) measures pain severity and pain interference in the last 24 h on 0–10 scales[28]. FACT-ES and DASH questionnaires were completed T0 and T3. BPI-SF scores were obtained T0, T1, T2, and T3.
Curcumin and estrogen analyses
Plasma curcumin levels were determined by direct and indirect enzymatic assays at T0 and T3 using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method[29]. For the indirect enzymatic assay, plasma samples underwent ß-glucuronidase hydrolysis of curcumin glucuronide by method of Vareed et al.[30] prior to LC-MS/MS analysis of curcumin.
An ultrasensitive LC-MS/MS method in the clinically relevant sub-picomolar range for E1 and E2 was developed and validated by procedure of Bertelsen et al.[31]. Detailed sample preparation procedures and LC–MS/MS analytical settings and operating modes are provided in Supplementary Information for quantitative curcumin, E1 and E2 analyses.
Statistical analysis
The primary goal of this pilot study was to evaluate the feasibility of using three validated instruments for evaluation of joint and other treatment-related symptoms in women on AI therapy taking NEC vs placebo in a randomized double-blind placebo-controlled trial. Sample size of 20 per group produces a two-sided 95% confidence interval with distance of 0.64 from the difference in means to the limits assuming a standard deviation (SD) of 1.0. Generalized Estimating Equation (GEE) was used to analyze the serially measured data for FACT-ES, DASH, BPI-SF using the compound symmetry working covariance matrix to account for within-person correlations across time and treating time as categorical. The interaction of treatment group by time was included in the GEE to test for differences in treatment effect over time. All randomized participants with at least one measurement were included in the GEE analysis.
For participants with measurements at T0 and T3, we calculated change scores (differences) between T3 and T0 (T3-T0) for FACT-ES, DASH, and BPI-SF and used the two-sample t-test to compare treatments. Following Hershman et al.[8], BPI-SF measurements at T1, T2, and T3 were considered clinically improved compared with T0 measurement for differences of ≥ 2 points. To compare the proportion of patients showing improvement across time between treatments, GEE for binary outcome using a compound symmetry working covariance matrix was used. Analyses used SAS 9.4.
Grip strength measurements for the two groups were compared using the two-sample t-test. Adherence was calculated by dividing the number of pills taken for days on study treatment by the number of pills dispensed for the days on study treatment.
Results
Study participants
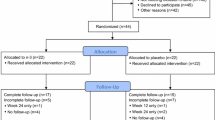
From 4/2019 to 1/2021, 42 eligible postmenopausal women with ER positive BC and AIIA were randomized to NEC or matched placebo capsules for the 3-month study (Fig. 1). There were eight dropouts following enrollment/randomization (Fig. 1), resulting in 34 (81% of 42) participants (n = 15 placebo, n = 19 NEC) with evaluable measurements at both baseline and 3-month timepoints (81% total enrolled, 87% T0 participants). Table 1 provides the demographic details of the 42 randomized participants.
Adherence
Based on pill counts and daily logs, average adherence to NEC/placebo for the 3-month study period was 92 ± 8.8% (n = 33 participants with one or both measures of adherence) and 90 ± 11.4% and 94 ± 5.8% in the placebo and NEC groups, respectively.
Adverse events
No clinically significant changes were observed from T0 to T3 for CBC and ALT (n = 32). Common grade 1 adverse events (AE) reported by patients included diarrhea, nausea, heartburn, headache, stool discoloration, flatulence, abdominal pain, constipation, dyspepsia, and belching (Table 2). A total of six patients reported grade 2 AE that included headache, heartburn, and dyspepsia. One participant was diagnosed with bone metastasis; this serious AE was not attributed to the study intervention.
PROMs
Of 42 participants enrolled, 39 initiated NEC/placebo with PROMs at T0 (93%) and 34/39 at both T0-T3 (87%).
FACT-ES
Higher FACT-ES scores indicate better well-being and fewer symptoms. Treatment effect was significant in GEE analysis (p = 0.039), with higher FACT-ES scores in the placebo group compared to NEC group at T3 (Fig. 2a, Table 3). Although FACT-ES subscale scores for physical and social/family well-being and endocrine symptoms did not change significantly by treatment, scores for emotional well-being (p = 0.03) and functional well-being (p = 0.04) significantly increased in the placebo relative to NEC groups (Table 3).
For the 34 participants (n = 19 NEC, n = 15 placebo) with evaluable data at both T0 and T3, mean total FACT-ES scores (Table 4) at T0 did not significantly differ by study arm (placebo mean (M) = 135.8, SD = 19.8 vs NEC M = 127.4, SD = 21.8, p = 0.25) by two-sample t-test. However, the placebo versus NEC group had a significantly higher mean score at T3 (placebo M = 150.9, SD = 20.2, NEC M = 135.6, SD = 20.5; p = 0.038).
The average change scores of FACT-ES did not differ significantly by treatment arms (p = 0.11, Table 4). The mean FACT-ES change score showed an improvement from T0 to T3 in both study arms, which was significant in the placebo group (Mean change = 15.0, SD = 12.3, p < 0.01) but not in the NEC group (Mean change = 7.1, SD = 15.6, p = 0.08). FACT-ES change scores for the individual subscales did not differ significantly by study arm (data not shown).
DASH
A higher DASH score indicates a greater symptom burden. Treatment difference was not significant in GEE analysis (p = 0.43) (Fig. 2b, Table 3). For the thirty participants (placebo n = 13, NEC n = 17) with evaluable data at T0 and T3, mean baseline DASH scores were 20.6 (SD = 15.4) and 24.2 (SD = 17.4) for the placebo and NEC groups, respectively (p = 0.74). A comparison of the mean change scores by treatment arm did not show a significant difference (p = 0.42, Table 4). Symptom burden did not change between T3 and T0 for participants in NEC (mean change = -6.9, SD = 14.05, n = 17, p = 0.06) or placebo (mean change = -3.3, SD = 8.7, n = 13, p = 0.20) groups.
BPI-SF
The BPI-SF measures pain intensity (severity) and the impact of pain on functioning (interference) for the past 24 h. Pain severity is measured via four items (pain at its worst, pain at its least, pain on the average, pain right now) and interference by how much pain has disrupted seven daily activities (general activity, walking, work, mood, enjoyment of life, relations with others, sleep). Treatment difference over time for BPI severity as a composite of the four pain items (mean severity score) was not significant in GEE analysis (p = 1.0) (Fig. 2c, Table 3). Similarly, BPI interference as a mean of the seven interference items did not differ significantly by treatment arm in GEE analysis (Fig. 2d, Table 3).
For those with data at T0 and T3, mean T0 BPI severity scores were 3.8 (SD = 1.7, n = 15) and 4.2 (SD = 1.8, n = 19) for placebo and NEC groups, respectively (Table 4). For these women, mean BPI severity scores decreased significantly from T0 to T3 for NEC (mean change score of -1.5, SD = 2.1, n = 19, p < 0.01) and for placebo (mean change score of -1.4, SD = 1.9, n = 15, p = 0.01). The mean change scores between T3 and T0 did not differ significantly between study arms (p = 0.88).
For participants with T0 and T3 data, mean baseline BPI interference scores were 3.1 (SD = 2.4, n = 15) and 3.3 (SD = 1.9, n = 19) for placebo and NEC groups, respectively (Table 4). Mean BPI Interference scores decreased significantly from T0 to T3 in the placebo arm (mean change score of − 1.5, SD = 1.3, p < 0.01) but not in the NEC arm (mean change score of − 1.0, SD = 2.2, p = 0.07). The mean change scores between T3 and T0 did not differ significantly between study arms (p = 0.37).
Using a positive two-point change in BPI score as the threshold for clinically meaningful improvement[8], the proportion of women with ≥ 2-point improvement at T1, T2, T3 compared to T0 did not change significantly for BPI severity (p = 0.33) or BPI interference (p = 0.08); no treatment group differences in trends were found (p > 0.5) for BPI severity and interference. Nevertheless, we noted a nonsignificant increase in the percentage of participants with improvement in BPI severity at T2, and T3 compared to T0 for the NEC compared to placebo study group (Fig. 3a). Examining improvement in terms of the individual items of the BPI Severity scoring, scores for pain at its least within the last 24 h followed a nonsignificant increasing trend in the percent of patients with improvement at T1 to T3 (Fig. 3b).
Of the participants who completed the 3-month intervention, 10 of 19 (53%) participants in the NEC group compared to 6 of 15 (40%) in the placebo arm had BPI severity scores improved by ≥ 2 points (p = 0.51). For BPI Interference, 7 of 19 (37%) and 7 of 15 (47%) participants in the NEC and placebo groups, respectively, had ≥ 2 point improvement (p = 0.73).
Grip strength
Twenty-four participants underwent grip strength testing at T0 and T3; measurements obtained with the same dynamometer (Jamar or Trialite) at both timepoints were analyzed (n = 21). Baseline mean grip strength measurements for right and left hands were 18.6 (SD = 5.8) kg and 17.8 (SD = 6.5) kg, respectively, for NEC (n = 12) and 18.3 (SD = 4.7) kg and 19.7 (SD = 8.1) kg, respectively, for placebo (n = 9). At T3, average right and left grip strength measurements were 20.6 (SD = 6.8) and 21.1 (SD = 8), respectively, for NEC and 18.5 (SD = 4.9) and 16.9 (SD = 6.1), respectively, for placebo ( Fig. 4a); there were no statistically significant differences by paired Student’s t-test within or between study groups (p > 0.14). T0–T3 changes in grip strength varied by individuals, with increased and decreased measurements in both groups (Fig. 4b), without significant differences by treatment arm (right p = 0.44, left p = 0.07). Combining results of left and right grip strength change, more participants had increases > 2 kg in the NEC versus placebo group (12 readings, n = 7 NEC vs 6 readings, n = 4 placebo, p = 0.05).
Plasma curcumin
Blood samples were obtained from 32/34 T0-T3 participants. We determined curcumin plasma levels in participants with T3 samples obtained ≤ 2 days of the last dose of study agent (n = 14 NEC, n = 9 placebo). Curcumin was not detected in baseline plasma samples of either study group by direct or indirect methods. Following β-glucuronidase hydrolysis, curcumin was detected in all NEC samples at T3 from 2.56 to 419 nM, with an average level of 148 ± 127.1 nM (Fig. 5). None of the study participants in the placebo group had detectable T3 plasma curcumin following β-glucuronidase hydrolysis (Fig. 5). Analysis of study capsules (3 each) two years following manufacture confirmed curcumin content at the expected levels (data not shown).
Serum E1 and E2
Serum E1 levels remained unchanged or below the lower limit of quantitation (LLoQ) at T0 and T3 in both study arms (n = 14 NEC, n = 9 placebo). Serum E2 decreased in two placebo arm patients, from 302.9 pmol/L and 107.6 pmol/L at baseline, respectively, to < LLoQ (< 3.67 pmol/L) at T3; E2 was otherwise unchanged < LLoQ from T0-T3.
Discussion
With this first randomized controlled, double-blind pilot trial of curcumin in postmenopausal women experiencing AIIA, we demonstrated feasibility of this multi-site study design by the following measures: (1) 87% completion of PROMs at T0-T3 (34/39 participants with T0); (2) 82% collection of lab/biospecimens at T0-T3 (32/39 participants with T0); (3) adherence > 90% in both arms; (4) accrual in 20 months with 87% T0 visits within 11 months until March 2020 and ensuing COVID19 pandemic restrictions on in-person clinical encounters.
Both study agents were well-tolerated, without attributable serious AEs. Gastrointestinal symptoms were the most common AE, which is consistent with reports of other trials using a variety of different curcumin formulations[18].
While this pilot trial was not powered to test for efficacy, we conducted analyses for preliminary estimates of differences between the two treatments. For the 34 participants with paired 0–3-month assessments, changes in PROM scores at T3 did not differ significantly between NEC and placebo; both arms had higher scores at T3 vs baseline. No adjustments were made for multiple tests for this pilot study; the p-value (p = 0.039) for FACT-ES did not account for multiple tests. As the placebo contained only excipients/food dyes, the greater improvement in FACT-ES and BPI-Interference scores in women taking placebo vs NEC was unexpected (Table 3). Increasing public awareness of the potential benefits of curcumin/turmeric based on traditional and Ayurvedic medicine contributed to patient interest in this trial and might have affected the reported outcomes with placebo[32].
We observed that BPI-Severity scores for the last 24 h, using BPI-SF, might not accurately capture the experience of joint arthropathy for our patient cohort where most women described waxing and waning symptoms with good and bad days. Notably, we observed a non-significant trend for the BPI severity component of least pain in last 24 h when analyzing for ≥ 2 point improvement. A more nuanced assessment of pain with BPI Long Form (e.g., pain over a week) or Penn Arthralgia score[33] might refine our ability to evaluate AIIA and treatment efficacy in future studies.
By grip strength, we noted a trend toward improvement with NEC relative to placebo treatment. The number of participants with pre- and post-intervention dynamometer readings was limited by the COVID19 pandemic, with decreased clinic access and patient preferences to avoid in-person follow-up in 2020 and 2021.
For this pilot project, plasma curcumin by enzymatic hydrolysis provided a biomarker of exposure, detected only in the NEC group samples. Most curcumin pharmacokinetic studies involve hydrolyzing plasma samples prior to quantitation due to the rapid metabolism of curcumin. Currently, there are no published pharmacokinetic studies evaluating the drug-drug interaction of curcumin and AIs. Our preliminary data on the lack of increased E1 and E2 serum levels suggest that curcumin may not enhance AI metabolism. We plan to correlate optimal curcumin dose with treatment effects in future studies.
Randomized controlled trials have demonstrated beneficial effects of curcumin for a wide range of clinical indications, including reduced osteoarthritis pain[13, 34, 35], improved memory and attention[19], decreased depressive symptoms[15], and anti-diabetic[36] and lipid-lowering[20] properties. Our limited study sample size was not powered for efficacy. Higher doses and longer duration of treatment with NEC might also be needed for amelioration of AIIA, despite reports of short-term improvement in osteoarthritis patients treated with curcumin[35, 37]. In addition, the COVID19 pandemic contributed to the lower completion rate of certain study endpoints, such as blood draws and grip strength measurements.
Taken together, this pilot study demonstrated the feasibility of a randomized double-blind, placebo-controlled trial of curcumin in women experiencing AIIA. We observed potential trends for improvements specific to NEC vs placebo in BPI-SF severity subscores and grip strength, with plasma curcumin as a viable marker of exposure. These study results provide the foundation for a future clinical trial to evaluate NEC for efficacy and safety in ameliorating AIIA.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available to protect study participant privacy.
Abbreviations
- AE:
-
Adverse events
- AIs:
-
Aromatase inhibitors
- AIIA:
-
Aromatase inhibitor-induced arthralgia
- ALT:
-
Alanine aminotransferase
- BC:
-
Breast cancer
- BPI-SF:
-
Brief Pain Inventory short form
- CBC:
-
Complete blood count
- DASH:
-
Disabilities of the shoulder, arm, and hand
- E1:
-
Estrone
- E2:
-
Estradiol
- ER:
-
Estrogen receptor
- FACT-ES:
-
Functional assessment of cancer treatment-endocrine symptoms
- GEE:
-
Generalized estimation equation
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- LLoQ:
-
Lower limit of quantitation
- M:
-
Mean
- NEC:
-
Nanoemulsion curcumin
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- PR:
-
Progesterone receptor
- PROMs:
-
Patient-reported outcome measures
- SD:
-
Standard of deviation
References
Goss PE et al (2016) Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 375(3):209–219. https://doi.org/10.1056/NEJMoa1604700. ((in eng))
Goss PE et al (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97(17):1262–1271. https://doi.org/10.1093/jnci/dji250. ((in eng))
Goss PE et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 349(19):1793–1802. https://doi.org/10.1056/NEJMoa032312. ((in eng))
Lombard JM et al (2016) Aromatase inhibitor induced musculoskeletal syndrome: a significant problem with limited treatment options. Supp Care Cancer 24(5):2139–2146. https://doi.org/10.1007/s00520-015-3001-5
Henry NL et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30(9):936–942. https://doi.org/10.1200/jco.2011.38.0261. ((in eng))
Henry NL, Unger JM, Till C, Crew KD, Fisch MJ, Hershman DL (2021) Predictors of pain reduction in trials of interventions for aromatase inhibitor-associated musculoskeletal symptoms. JNCI Cancer Spectr. https://doi.org/10.1093/jncics/pkab087. ((in eng))
Henry NL et al (2018) Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol 36(4):326–332. https://doi.org/10.1200/jco.2017.74.6651. ((in eng))
Hershman DL et al (2018) Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA 320(2):167–176. https://doi.org/10.1001/jama.2018.8907. ((in eng))
Hershman DL et al (2015) Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol 33(17):1910–1917. https://doi.org/10.1200/jco.2014.59.5595. ((in eng))
Shen S et al (2018) Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res Treat 172(3):603–610. https://doi.org/10.1007/s10549-018-4946-0. ((in eng))
Menon VP, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 595:105–125. https://doi.org/10.1007/978-0-387-46401-5_3. ((in eng))
Young NA et al (2014) Oral administration of nano-emulsion curcumin in mice suppresses inflammatory-induced NFκB signaling and macrophage migration. PLoS ONE 9(11):e111559. https://doi.org/10.1371/journal.pone.0111559. ((in eng))
Belcaro G et al (2010) Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev 15(4):337–344 ((in eng))
Daily JW, Yang M, Park S (2016) Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food 19(8):717–729. https://doi.org/10.1089/jmf.2016.3705. ((in eng))
Lopresti AL, Drummond PD (2017) Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: a randomised, double-blind, placebo-controlled study. J Affect Dis 207:188–196. https://doi.org/10.1016/j.jad.2016.09.047
Cox KHM, White DJ, Pipingas A, Poorun K, Scholey A (2020) Further evidence of benefits to mood and working memory from lipidated curcumin in healthy older people: a 12-week double-blind placebo-controlled partial replication study. Nutrients. https://doi.org/10.3390/nu12061678. ((in eng))
Bergman J et al (2013) Curcumin as an add-on to antidepressive treatment: a randomized, double-blind, placebo-controlled, pilot clinical study. Clin Neuropharmacol 36(3):73–7. https://doi.org/10.1097/WNF.0b013e31828ef969. ((in eng))
Tsai IC, Hsu CW, Chang CH, Tseng PT, Chang KV (2021) The effect of curcumin differs on individual cognitive domains across different patient populations: a systematic review and meta-analysis. Pharmaceuticals (Basel). https://doi.org/10.3390/ph14121235. ((in eng))
Small GW et al (2018) Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry 26(3):266–277. https://doi.org/10.1016/j.jagp.2017.10.010. ((in eng))
Panahi Y, Khalili N, Hosseini MS, Abbasinazari M, Sahebkar A (2014) Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: Results of a randomized controlled trial. Complement Ther Med 22(5):851–857. https://doi.org/10.1016/j.ctim.2014.07.006. ((in eng))
Pouton CW (2000) Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci 11(Suppl 2):S93–S98. https://doi.org/10.1016/s0928-0987(00)00167-6. ((in eng))
Dressman JB, Reppas C (2000) In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci 11:S73–S80. https://doi.org/10.1016/S0928-0987(00)00181-0
Zhongfa L et al (2012) Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol 69(3):679–689. https://doi.org/10.1007/s00280-011-1749-y. ((in eng))
Irwin ML et al (2015) Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 33(10):1104–1111. https://doi.org/10.1200/jco.2014.57.1547. ((in eng))
Crew KD et al (2010) Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol 28(7):1154–1160. https://doi.org/10.1200/JCO.2009.23.4708
Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55(2):189–199. https://doi.org/10.1023/a:1006263818115. ((in eng))
Hudak PL, Amadio PC, Bombardier C (1996) Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected], the upper extremity collaborative group (UECG). Am J Ind Med 29(6):602–608. https://doi.org/10.1002/(sici)1097-0274(199606)29:6%3C602::aid-ajim4%3E3.0.co;2-l. ((in eng))
Daut RL, Cleeland CS, Flanery RC (1983) Development of the wisconsin brief pain questionnaire to assess pain in cancer and other diseases. Pain 17(2):197–210. https://doi.org/10.1016/0304-3959(83)90143-4. ((in eng))
Chen W et al (2012) A liquid chromatography-tandem mass spectrometric method for quantification of curcumin-O-glucuronide and curcumin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 900:89–93. https://doi.org/10.1016/j.jchromb.2012.05.026. ((in eng))
Vareed SK et al (2008) Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 17(6):1411–1417. https://doi.org/10.1158/1055-9965.Epi-07-2693. ((in eng))
Bertelsen BE et al (2020) An ultrasensitive routine LC-MS/MS method for estradiol and estrone in the clinically relevant sub-picomolar range. J Endocr Soc 4(6):47. https://doi.org/10.1210/jendso/bvaa047. ((in eng))
Hróbjartsson A, Gøtzsche PC (2001) Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 344(21):1594–1602. https://doi.org/10.1056/nejm200105243442106. ((in eng))
Brier MJ, Chambless DL, Lee L, Mao JJ (2015) Development and validation of the penn arthralgia aging scale among breast cancer survivors. Cancer 121(16):2808–2813. https://doi.org/10.1002/cncr.29407. ((in eng))
Zeng L, Yu G, Hao W, Yang K, Chen H (2021) The efficacy and safety of Curcuma longa extract and curcumin supplements on osteoarthritis: a systematic review and meta-analysis. Biosci Rep. https://doi.org/10.1042/bsr20210817. ((in eng))
Nakagawa Y et al (2014) Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci 19(6):933–939. https://doi.org/10.1007/s00776-014-0633-0. ((in eng))
Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S (2012) Curcumin extract for prevention of type 2 diabetes. Diabetes Care 35(11):2121–2127. https://doi.org/10.2337/dc12-0116. ((in eng))
Belcaro G et al (2010) Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med 52(2 Suppl 1):55–62 ((in eng))
Acknowledgements
We thank all the study participants, clinicians, clinic staff, and clinical trial teams involved in the study. This pilot project was funded by the Alliance NCORP Research Base – pilot project pilot grant award UG1 CA189823-04 (LDY, ML).
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1 CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1 CA233331, and UG1 CA233337. https://acknowledgments.alliancefound.org. Correlative analyses reported in this paper included work performed in the Pharmacoanalytical Shared Resource of The Ohio State University Comprehensive Cancer Center and Analytical Pharmacology Shared Resource of the City of Hope Comprehensive Cancer Center, which are supported by Cancer Center Support Grants P30CA016058 and P30CA033572, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
LDY, ML, and PFH contributed to study conception and design. LDY, CK, MSS, ML, and LW developed the protocol. LW served as biostatistician for the trial. Quantitative analyses were performed by KH, MP for curcumin and TS, YH for estradiol. LW, LDY, PFH, ML, and NT contributed to data analysis and interpretation. LDY and ML provided oversight for running the clinical trial and project administration. KWH contributed to recruitment, data collection, biospecimen collection. All authors contributed to manuscript preparation, writing, and/or revising the draft and approving the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
All patients provided written informed consent before participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lustberg, M., Fan-Havard, P., Wong, F.L. et al. Randomized placebo-controlled, double-blind clinical trial of nanoemulsion curcumin in women with aromatase inhibitor-induced arthropathy: an Alliance/NCORP pilot trial. Breast Cancer Res Treat 205, 61–73 (2024). https://doi.org/10.1007/s10549-023-07223-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07223-4