Abstract
Purpose
Cancer-related cognitive impairment (CRCI) following chemotherapy is commonly reported in breast cancer survivors, even years after treatment. Data from preclinical studies suggest that exercise during chemotherapy may prevent or diminish cognitive problems; however, clinical data are scarce.
Methods
This is a pragmatic follow-up study of two original randomized trials, which compares breast cancer patients randomized to exercise during chemotherapy to non-exercise controls 8.5 years post-treatment. Cognitive outcomes include an online neuropsychological test battery and self-reported cognitive complaints. Cognitive performance was compared to normative data and expressed as age-adjusted z-scores.
Results
A total of 143 patients participated in the online cognitive testing. Overall, cognitive performance was mildly impaired on some, but not all, cognitive domains, with no significant differences between groups. Clinically relevant cognitive impairment was present in 25% to 40% of all participants, regardless of study group. We observed no statistically significant effect of exercise, or being physically active during chemotherapy, on long-term cognitive performance or self-reported cognition, except for the task reaction time, which favored the control group (β = -2.04, 95% confidence interval: -38.48; -2.38). We observed no significant association between self-reported higher physical activity levels during chemotherapy or at follow-up and better cognitive outcomes.
Conclusion
In this pragmatic follow-up study, exercising and being overall more physically active during or after adjuvant chemotherapy for breast cancer was not associated with better tested or self-reported cognitive functioning, on average, 8.5 years after treatment. Future prospective studies are needed to document the complex relationship between exercise and CRCI in cancer survivors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decades, the number of individuals living with and beyond a breast cancer diagnosis has increased [1,2,3]. Projections forecast that the population of cancer survivors will continue to grow in future years [4]. In this context, adequate care of cancer (therapy)-related side effects is increasingly important.
Cancer-related cognitive impairment (CRCI) is among the most common and burdensome side-effects in both breast cancer patients and survivors. The prevalence of CRCI varies widely across studies, with a mean prevalence of 44% for self-reported CRCI [5]. Prior research has reported that effects on cognitive performance can be detected even 20 years after treatment [6]. The pathophysiology of CRCI is multifactorial, with key roles for (anthracycline-based) chemotherapy, having cancer itself, and co-existing fatigue [5]. Depression and anxiety are also strongly correlated with cognitive problems [7]. In most patients, complaints of CRCI are mild to moderate [8], yet they can profoundly impact the quality of life [9]. Although some interventions are promising, no strategy is currently widely implemented or accepted to prevent CRCI in breast cancer patients [10].
Physical exercise during chemotherapy has been proposed as a strategy to prevent CRCI. Rodent studies describe various pathways via which exercise can benefit cognition, such as stimulating hippocampal neurogenesis [11, 12]. In non-cancer populations, most, but not all [13] studies report an association between higher levels of physical activity [14, 15] or exercise interventions [16, 17] and better cognitive outcomes. In cancer patients, most trials studied the effect of an exercise intervention after treatment (i.e., in survivors), with most of them reporting positive effects on perceived cognition and not on tested cognition [18,19,20,21]. One small, randomized study (N = 25 per study arm) suggests that an unsupervised, home-based walking intervention during chemotherapy might mitigate self-reported CRCI directly after treatment [22]. Evidence from larger, well-conducted trials with longer follow-up times is lacking.
In this study, we evaluated the effect of an aerobic and resistance exercise intervention during adjuvant chemotherapy for breast cancer on cognitive testing and self-reported cognitive complaints measured, on average, 8.5 years after treatment. We hypothesized that exercise during chemotherapy, relative to usual care control, results in less CRCI years after treatment.
Methods
Setting and participants
The current analysis is part of the Pact-Paces-Heart study, a follow-up investigation of two previously performed randomized controlled trials (RCTs): the Physical Activity during Cancer Treatment (PACT) study and the Physical exercise during Adjuvant Chemotherapy Effectiveness Study (PACES). The design and results of the Pact-Paces-Heart study on cardiovascular outcomes (submitted), as well as results of the original studies, have been published elsewhere [23,24,25]. In brief, the PACT and PACES studies were conducted between 2009–2013 and included 204 and 230 non-metastasized breast cancer patients, respectively. In the PACT study, participants were randomized to either a supervised, moderate-to high-intensity exercise intervention or a control group. The intervention started six weeks after diagnosis with a fixed duration of 18 weeks. PACES’ design was comparable, except that there was a second intervention arm (a home-based, low-intensity exercise program), and both interventions of PACES started with the first cycle of chemotherapy and continued until three weeks post-treatment. Both studies collected data (e.g., physical fitness, muscle strength, and patient-related outcomes, including quality of life) at baseline, at the end of chemotherapy, and approximately six months after baseline. In PACT, physical activity levels were recorded by the Short Questionnaire to assess Health-enhancing physical activity (SQUASH) [26]. PACES used the Physical Activity Scale for Elderly [27]. In the follow-up study, physical activity was assessed via the SQUASH. Information on the exercise intervention, including the exercise dose and adherence, is provided in Appendix.
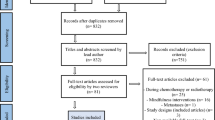
The parent study included 185 breast cancer survivors free of recurrent or metastasized cancer. Participants underwent physical measurements (i.e., cardiac MRI, cardiopulmonary exercise test) and completed questionnaires. Participation in additional cognitive testing was optional. A detailed description of the flow of participants through the studies is provided in Fig. 1. The study was approved by the UMC Utrecht institutional review board and was registered with the International Clinical Trial Registry Platform (identifier NTR7247). All patients provided written informed consent.
Cognitive outcomes
An overview of the data collection of cognitive outcomes at the different time points is provided in Table 1. Objective cognitive testing was performed using the online Amsterdam Cognition Scan (ACS). The ACS is a recently developed, self-administrated neuropsychological test battery that includes 11 computerized tests, based on traditional neuropsychological tests, in the following five cognitive domains: (1) learning and memory; (2) attention and working memory; (3) processing speed; (4) executive functioning; and (5) motor functioning [28]. Reliability and validity of the ACS have been previously described [28], and other oncology studies have used this tool to assess cognitive performance [20, 29].
Subjective cognitive complaints were assessed with the M.D. Anderson Symptom Inventory (MDASI) questionnaire [30], with additional questions from the MDASI multiple myeloma module [31. The cognitive questions of this module are not specific to multiple myeloma patients and have been previously related to tested cognition [32]. We included two questions on the severity of memory and attention problems and four questions on interference with daily life. Response options were on a 0–10 numeric scale, ranging from “not present” to “as bad as you can imagine” and “did not interfere” to “interfered completely” for the questions on severity and interference, respectively. From these raw scores, a mean subscale score for severity and interference was derived. A previous study reported good-to-excellent reliability, with Cronbach α coefficient values of 0.88 for the severity subscale and 0.91 for the interference subscale [31]. Also, in both the original studies and the follow-up study, all participants of the Pact-Paces-Heart study completed questionnaires on patient-reported outcomes, including the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) [33]. “This questionnaire included two questions on cognitive function”. Following the EORTC QLQ-C30 scorings manual, we calculated the score for the cognitive functional scale by averaging the item scores and linearly transforming this value to obtain a range from 0 to 100, where a higher score corresponds with better functioning.
Statistical analysis
Numerical data are presented as mean ± standard deviation, and ordinal data or numerical data violating normality assumptions as median [min–max]. Using pre-defined criteria, all ACS entries indicative of poor test understanding, periods of participant distraction, or computer/network problems were identified and excluded. For those tests where higher scores corresponded with worse cognitive performance, we calculated the absolute median deviation per age category (≤ 40, 41–59, ≥ 59 years), and all entries > 3.5 units were considered outliers and removed from the database [34]. To contextualize the overall cognitive performance of our cohort, ACS scores in our sample were compared to normative data (based on 248 healthy adult controls) [35], and expressed as age-adjusted Z-scores per study arm. For the cognitive functioning scale of EORTC QLQ-C30, we interpreted our results compared to previously described normative data [36] and used a score of < 75 as the threshold for clinically relevant cognitive impairment [37].
We used intention-to-treat regression models with treatment allocation (moderate-to high-intensity exercise versus control) and cognitive outcomes as independent and dependent variables, respectively. All outcomes were modeled linearly, except for those expressed on an ordinal scale (i.e., the correct number of words or sequences), which were modeled via a modified Poisson regression (with a log-link) and expressed as relative differences with robust standard errors (sandwich estimates). For the linear models with non-normally distributed residuals (e.g., the MDASI questionnaire data where most participants reported near-zero scores), estimates and bias-corrected confidence intervals (CIs) were calculated using a bootstrapped distribution based on 10,000 replications [38, 39].
All models were adjusted for age, education (low versus middle or high), study (PACT versus PACES), currently receiving endocrine treatment, and cumulative doxorubicin equivalent dosage (with ratio doxorubicin: epirubicin = 1: 0.7 [40]). We additionally adjusted these models for baseline EORTC QLQ C30 scores. Analyses were repeated with changes in self-reported physical activity during chemotherapy, independent of treatment allocation, as the main independent variable. Change in physical activity was defined as the level of physical activity after the intervention (T1) minus the level of physical activity at baseline (T0) and expressed as a z-score, given that the two original studies used different physical activity questionnaires. Restricted cubic splines were used to evaluate the potential nonlinearity of the latter models. We considered a p-value > 0.05 for the non-linear term as no indication of nonlinearity. Last, we repeated the analyses with physical activity levels at follow-up, expressed as minutes/week as the primary independent variable.
All exercise analyses were limited to the moderate-to high-intensity and control group because the low-intensity group of PACES was too small (N = 20). In a sensitivity analysis, we added data from these low intensity participants to the moderate-to-high intensity exercise group. The data from the low-intensity group was also included in the analyses with self-reported physical activity, given that those analyses were irrespective of randomization. All analyses were performed with R studio software (version 4.3.0, Rstudio Inc., Boston, MA).
Results
Of the 185 Pact-Paces-Heart study participants, 143 (N = 143/185; 77.3%) participated in the optional cognitive testing. The demographic characteristics of those who completed the cognitive testing were comparable to those who did not participate in the cognitive testing and the original study sample (Supplementary Table 1).
Descriptive results
Of the 143 participants, 66 had been allocated to the moderate-to high-intensity exercise program and 57 to the control arm. Characteristics of these participants is presented in Table 2. Information on the 20 participants of the low-intensity arm is provided in Supplementary Table 2.
The moderate-to high-intensity and control group were comparable in most characteristics (Table 2). The average age at the time of cognitive testing was 58 years, and the vast majority (> 90%) of the participants were post-menopausal. Half of the participants in the control arm and 56% of the women in the moderate-to high-intensity exercise group were highly educated. All participants, except one, received treatment with anthracyclines, with median doxorubicin (equivalent) dosages of 241 [91–420] mg/m2 and 235 [0–420] mg/m2, in the control and moderate-to high-intensity exercise group, respectively. At the time of cognitive testing, 11 (N = 11/57; 19.6%) control participants and 7 participants of the moderate-to high-intensity exercise program (N = 7/66; 10.8%) received endocrine therapy. Comorbidities were reported in 21 (N = 21/57; 37.5%) and 22 (N = 22/77; 33.8%), respectively.
Self-reported QLQ-C30 cognitive functioning scores are presented in Table 3. The median score before treatment was 83.3 [0–100.0] in the control and 83.3 [16.7–100.0] in the moderate-to high-intensity group, with self-reported impaired cognitive functioning in 24.6% (N = 14/57) and 39.4% (N = 26/66), respectively. After chemotherapy treatment, these percentages increased to 50.0%(N = 24/48) in the control arm and 53.0% (N = 35/66) in the moderate-to high-intensity exercise arm directly after the intervention. At the six-month follow-up, 40.4% (N = 21/52) of the control participants and 41.3% (N = 26/62) of the moderate-to high-intensity exercise group participants reported impaired cognitive function. At 8.5 years post-treatment, median scores were comparable between study arms; 83.3 [0–100.0] and 83.3 [16.7–100.0], respectively. However, at follow-up, more patients in the exercise group reached the threshold for cognitive impairment (N = 31/66; 47.0%) compared to control participants (N = 20/56; 35.7%).
Scores on the ACS and the MDASI 8.5 years post-treatment are presented in Table 4. Based on the age-adjusted z-scores, participants in our study tended to score lower than healthy controls on the tests assessing learning and memory, attention and working memory, and motor functioning. Above average, although with wide/ non-significant confidence intervals, z-scores were observed for tests of the domain’s processing speed and executive functioning.
For self-reported cognitive functioning (MDASI) 8.5 years after treatment, most patients reported none or mild symptoms with median scores of 1.50 [0–10] in control participants and 2.0 [0–9] in participants of the moderate-to high-intensity exercise program. Moderate or severe symptoms were reported by 6 (10.7%) and 5 (8.9%), 9 (13.6%), and 7 (10.6%) participants, respectively. Scores on the interference subscale were 0.33 [0–7] in the control group and 0.92 [0–7.3] in the moderate-to high-intensity exercise group.
Effect of moderate-to high-intensity exercise on long-term tested and perceived cognition
We did not find any significant effect of moderate-to high-intensity exercise during chemotherapy on objective cognitive testing 8.5 years after treatment (Table 5). The result for the test Reaction Time significantly favored the control group (β per 10-ms = 1.87, 95%CI: 0.06; 3.69). Estimates for self-reported cognitive functioning (MDASI) tended to favor control participants, although the results were not statistically significant. The models with additional correction for baseline EORTC QLQ-C30 cognitive functioning scores yielded comparable results, except for the results for the Reaction Time test (β per 10-ms = 1.83, 95%CI: -0.01; 3.67). In the sensitivity analysis, where data of participants of the low-intensity exercise program were added to the intervention group, the result on the test Reaction was not significant anymore; β per 10-ms = 1.70, 95%CI: -0.03; 3.43, while the test on Digit Sequence II now significantly favored the exercise arm (relative difference per number of sequences of 1.14, 95%CI: 1.02, 1.26). The other models generated similar conclusions to those presented in Table 4 (data not shown).
Association between physical activity levels and long-term cognition
An increase in physical activity level during chemotherapy was not associated significantly with objectively assessed or self-reported cognitive functioning years after treatment (Table 6). Similarly, physical activity levels at follow-up were not associated significantly with cognitive outcomes (relative differences range from 0.99 to 1.01, and beta coefficients per 0 to 0.07 per 10-min difference in reported physical activity, data not shown).
Discussion
In this follow-up study, we investigated the effect of exercise and physical activity on tested cognitive functioning and self-reported cognitive complaints in patients with breast cancer who had participated in one of two randomized clinical trials of exercise programs during their primary chemotherapy treatment approximately 8.5 years earlier. Overall, cognitive performance in some domains (particularly learning and memory) was mildly impaired compared to normative data. Most, but not all, participants reported low levels of perceived cognitive symptoms, with less interference in daily life. We observed no significant effects of moderate-to high-intensity exercise or being more physically active during chemotherapy on tested or perceived cognitive functioning years later, compared to non-exercise controls. Moreover, regardless of randomization, we found no significant association between those who reported higher physical activity levels at follow-up and (better) cognitive outcomes.
Most previous studies that have investigated the effects of exercise performed after the treatment of breast cancer on CRCI reported positive effects on (self-reported) cognitive outcomes [18,19,20] However, minimal evidence is available on the efficacy of exercise during chemotherapy on CRCI. From a mechanistic point of view, multiple pathways support the hypothesis of exercise-mediated neuroprotection, including increased resting brain-derived neurotrophic factor (important for various cellular processes such as neurogenesis), local changes in vascularization, and less neuroinflammation [41,42,43]. This biological rationale is supported by the results of a recent, large observational study of breast cancer patients undergoing adjuvant chemotherapy (N = 580), that found that higher self-reported physical activity levels before and during chemotherapy were associated with better perceived and objectively measured cognitive function after chemotherapy completion [44]. The only currently available trail that reports on the effectiveness of exercise during chemotherapy generated inconclusive results. This study randomizes breast cancer patients to either an unsupervised, home-based walking intervention during chemotherapy (N = 25) or usual care (N = 25) and found significantly higher levels of perceived cognitive complaints in the latter group but not in the exercise group [22]. There was some evidence for between-group differences (p interaction for study group x time: 0.05). Nevertheless, given the limited sample size and that objective cognitive functioning appeared to be unaffected, limited conclusions for clinical practice can be made from these results, and thus more robust evidence is needed.
Our results indicate that exercise during chemotherapy was not associated with tested CRCI years after treatment. This finding does not necessarily mean that there were no exercise effects directly after treatment, given that cognitive performance may change over time. The original PACES study reported an effect of exercise on self-reported cognitive complaints, based on the EORTC QLQ C-30, with an effect size of 0.33 [24]. A recent individual participant meta-analysis reported small effects of exercise post-treatment on self-reported cognitive functioning [45]. In a longitudinal, randomized study among breast cancer patients that studied the effects of self-affirmation(N = 160), perceived cognitive symptoms also varied over time. While the MDASI scores initially increased from baseline to the end of chemotherapy, at six months after chemotherapy, scores on the symptom subscale gradually decreased to an average of 2.10 ± 2.01 for patients in the control arm [46]. These findings have been corroborated by longitudinal neuroimaging studies documenting decreased cognitive performance during chemotherapy, with partial recovery [47] or even increased performance years after treatment in some patients [48]. Our study also observed above-average test scores in the domains of Processing Speed and Executive Functioning, but confidence intervals were wide. The current prevailing hypothesis is that the adult brain, although to a lesser extent than during childhood/adolescence, has the capacity to adapt to environmental changes and recover after disease by, for example, recruiting alternative neuronal circuits [49,50,51]. These neural plasticity processes are likely susceptible to cognitive training, such as memory training or speed tasks [52, 53]. If and to what extent participants compensated for cognitive impairments over time (with or without exercise) is an interesting topic for future research.
Based on the MDASI questionnaire, more than three-quarters of our study participants reported no or mild cognitive symptoms. Scores on the interference subscale were also low. Nevertheless, these results also indicate that a substantial proportion has moderate, or even severe, cognitive complaints years after treatment. The latter aligns with the findings on the EORTC QLQ-C30 questionnaire, in which 40% of the participants reached the threshold for clinically relevant cognitive impairment. The MDASI questionnaire assesses cognitive symptoms and their interference in the past 24 h, while the EORTC QLQ-C30 cognitive functioning is based on the past week. Subscales on the former instrument are also based on more questions with more extensive scoring ranges, which might have allowed for reporting more details on cognitive complaints. Prior research has reported a good (ρ = 0.69) and a moderate correlation (ρ = 0.49) between the MDASI symptom and interference subscale and the EORTC QLQ-C30 cognitive functioning scale, respectively [31].
Our current study was designed as a post-hoc, post-trial follow-up (FU) investigation of two original randomized trials (i.e., the PACT and PACES study). This post-trial FU design allowed for pragmatically investigating the effect of exercise and physical activity on long-term CRCI in a relatively large sample of breast cancer survivors. Post-trial FU studies can effectively detect persistent or even enhanced treatment effects years after completion of the original trials, sometimes referred to as the ‘legacy effect’ [54, 55]. Also, delayed adverse effects, which take years or even decades to become clinically apparent, can be detected by post-trial FU [56], as exemplified by former studies documenting the cardiotoxic properties of high-dose thoracic radiotherapy [57,58,59]. However, by design, post-trial FU studies may be susceptible to selective response/drop-out, especially with more extended periods of FU. In the context of our research, it is conceivable that breast cancer survivors who were originally randomized to the intervention program, or controls who are currently relatively fit and free of symptoms, were more willing to participate in our follow-up trial (and especially in additional, optional cognitive tests). Indeed, we included slightly more participants who were originally randomized to the exercise arm than to the control arm; 46% (n = 66/143) versus 40% (n = 57/143), respectively. The proportion of control participants in this follow-up study with cognitive impairment before treatment was lower (n = 14/57; 24.6%) than the proportion in the exercise group (n = 26/66; 39.4%). Thus, a selective response may have diluted our results to a certain extent, although we observed no significant difference in demographic characteristics between those who participated in our FU study and those who did not. A recently published systematic review recommended using registries and data linkage as the most effective approach for post-trial FU studies [56]. Given that such an approach is not possible for endpoints such as patient-reported outcome measures, we suggest, as we did in the first follow-up of PACT [25], that future randomized studies embed a question in the informed consent that allows for potential future data linkage and study invitation to facilitate future post-trial FU investigations.
Our study has several strengths and limitations in addition to those related to the post-trial FU design. A strength is the combination of objectively tested and self-reported cognitive outcomes in our study, given that these outcomes often are not highly correlated and might measure different constructs of CRCI [60, 61]. An additional limitation is that, apart from the EORTC QLQ-C30 questionnaire, cognitive outcomes were not included in the original trials, and thus we cannot correct for baseline values of the outcome. Also, not all participants experienced cognitive impairment prior to the intervention, with half of them reaching the threshold for clinically impaired cognitive functioning (Table 3). This means that the other half was unlikely to benefit from the intervention, which presumably limited our ability to study the effectiveness of the exercise program on cognitive functioning. Last, our exercise program was not tailored specifically to address cognitive complaints and was perhaps not the most optimal program for that purpose. A previous meta-analysis indicated that, in addition to aerobic and resistance exercise, non-western traditional modes of exercise, such as Tai Chi or yoga, are at least equally effective in improving cognitive functioning [62]. Thus, a multicomponent exercise program incorporating more holistic exercises might confer greater improvement in cognition functioning.
In conclusion, in this pragmatic follow-up study, we observed that exercising or being more physically active during chemotherapy was not associated with tested and perceived cognitive functioning years after treatment for breast cancer. Similarly, higher levels of reported physical activity at follow-up were not associated with better cognitive outcomes. Future prospective studies are warranted to investigate the complex relationship between exercise and enhanced physical activity among breast cancer patients who have experienced treatment-related impairment in their cognitive functioning.
Data availability
The data supporting this study’s findings are available from the corresponding author, AM, upon reasonable request.
References
Miller KD et al (2016) Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 66(4):271–289. https://doi.org/10.3322/caac.21349
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Kocarnik JM et al (2022) Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol 8(3):420–444. https://doi.org/10.1001/jamaoncol.2021.6987
Cancer Treatment | Survivor Facts & Figures. https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed 21 Mar 2023
Whittaker AL, George RP, O’Malley L (2022) Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: a systematic review and meta-analysis. Sci Rep 12(1). https://doi.org/10.1038/S41598-022-05682-1
Van Der Willik KD, Koppelmans V, Hauptmann M, Compter A, Ikram MA, Schagen SB (2018) Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res 20(1). https://doi.org/10.1186/S13058-018-1062-3.
Fardell JE, Irwin CM, Vardy JL, Bell ML (2023) Anxiety, depression, and concentration in cancer survivors: National Health and Nutrition Examination Survey results. Support Care Cancer 31(5). https://doi.org/10.1007/S00520-023-07710-W
Wefel JS, Kesler SR, Noll KR, Schagen SB (2015) Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin 65(2):123–138. https://doi.org/10.3322/CAAC.21258
Ahles TA, Root JC (2018) Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol 14:425–451. https://doi.org/10.1146/ANNUREV-CLINPSY-050817-084903
Schagen SB, Tsvetkov AS, Compter A, Wefel JS (2022) Cognitive adverse effects of chemotherapy and immunotherapy: are interventions within reach? Nat Rev Neurol 18(3):173–185. https://doi.org/10.1038/S41582-021-00617-2
Liu PZ, Nusslock R (2018) Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci 12. https://doi.org/10.3389/FNINS.2018.00052
Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H (2011) Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem 18(9):605–609. https://doi.org/10.1101/LM.2283011
Lenze EJ et al (2022) Effects of mindfulness training and exercise on cognitive function in older adults: a randomized clinical trial. JAMA 328(22):2218–2229. https://doi.org/10.1001/JAMA.2022.21680
Erickson KI et al (2019) Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc 51(6):1242–1251. https://doi.org/10.1249/MSS.0000000000001936
Prakash RS, Voss MW, Erickson KI, Kramer AF (2015) Physical activity and cognitive vitality. Annu Rev Psychol 66:769–797. https://doi.org/10.1146/ANNUREV-PSYCH-010814-015249
Falck RS, Davis JC, Best JR, Crockett RA, Liu-Ambrose T (2019) Impact of exercise training on physical and cognitive function among older adults: a systematic review and meta-analysis. Neurobiol Aging 79:119–130. https://doi.org/10.1016/J.NEUROBIOLAGING.2019.03.007
Biazus-Sehn LF, Schuch FB, Firth J, de Stigger FS (2020) Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: a systematic review and meta-analysis. Arch Gerontol Geriatr 89. https://doi.org/10.1016/J.ARCHGER.2020.104048
Campbell KL, Zadravec K, Bland KA, Chesley E, Wolf F, Janelsins MC (2020) The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys Ther 100(3):523–542. https://doi.org/10.1093/PTJ/PZZ090
Ren X et al (2022) Effects of physical exercise on cognitive function of breast cancer survivors receiving chemotherapy: a systematic review of randomized controlled trials. Breast : Official J Eur Soc Mastol 63:113. https://doi.org/10.1016/J.BREAST.2022.03.014
Koevoets EW et al (2022) Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomized controlled trial (PAM study). Breast Cancer Res 24(1). https://doi.org/10.1186/S13058-022-01530-2
Gehring K et al (2020) A pilot randomized controlled trial of exercise to improve cognitive performance in patients with stable glioma: a proof of concept. Neuro Oncol 22(1):103–115. https://doi.org/10.1093/NEUONC/NOZ178
Gokal K, Munir F, Ahmed S, Kancherla K, Wallis D (2018) Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy? Results from a small randomised controlled trial. PLoS One 13(11). https://doi.org/10.1371/JOURNAL.PONE.0206874
Travier N et al (2015) Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med 13:121. https://doi.org/10.1186/s12916-015-0362-z
van Waart H et al (2015) Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 33(17):1918–1927. https://doi.org/10.1200/JCO.2014.59.1081
Witlox L et al (2018) Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Med 16(1):86. https://doi.org/10.1186/s12916-018-1075-x
Wendel-Vos GCW, Schuit AJ, Saris WHM, Kromhout D (2003) Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 56(12):1163–1169. https://doi.org/10.1016/s0895-4356(03)00220-8
Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA (1999) The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol 52(7):643–651. https://doi.org/10.1016/s0895-4356(99)00049-9
Feenstra HEM, Murre JMJ, Vermeulen IE, Kieffer JM, Schagen SB (2018) Reliability and validity of a self-administered tool for online neuropsychological testing: the Amsterdam Cognition Scan. J Clin Exp Neuropsychol 40(3):253–273. https://doi.org/10.1080/13803395.2017.1339017
Klaver KM et al (2022) Neuropsychological test performance and self-reported cognitive functioning associated with work-related outcomes in occupationally active cancer survivors with cognitive complaints. J Cancer Surviv. https://doi.org/10.1007/S11764-022-01223-X
Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC (2000) Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 89(7):1634–1646
Jones D et al (2013) Validation of the M. D. Anderson Symptom Inventory multiple myeloma module. J Hematol Oncol 6(1). https://doi.org/10.1186/1756-8722-6-13
Jones D, Vichaya EG, Wang XS, Sailors MH, Cleeland CS, Wefel JS (2013) Acute cognitive impairment in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplant. Cancer 119(23):4188–4195. https://doi.org/10.1002/CNCR.28323
Aaronson NK et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376. https://doi.org/10.1093/JNCI/85.5.365
Leys C, Klein O, Bernard P, Licata L (2013) Detecting outliers: DO not use standard deviations around the mean, do use the median absolute deviation around the median. J Exp Soc Psychol 4:764–766
Feenstra HEM, Vermeulen IE, Murre JMJ, Schagen SB (2018) Online self-administered cognitive testing using the Amsterdam cognition scan: establishing psychometric properties and normative data. J Med Internet Res 20(5). https://doi.org/10.2196/JMIR.9298
de Ligt KM, Aaronson NK, Liegl G, Nolte S (2023) Updated normative data for the EORTC QLQ-C30 in the general Dutch population by age and sex: a cross-sectional panel research study. Qual Life Res. https://doi.org/10.1007/S11136-023-03404-2
Giesinger JM et al (2020) Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J Clin Epidemiol 118:1–8. https://doi.org/10.1016/J.JCLINEPI.2019.10.003
Bland JM, Altman DG (2015) Statistics Notes: bootstrap resampling methods. BMJ 350. https://doi.org/10.1136/BMJ.H2622
A. R. C. to A. R. T. E. S. (2019) Functions to accompany J. Fox and S. Weisberg, “car: Companion to Applied Regression.”
Zamorano JL et al (2016) 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (E. Eur Heart J 37(36):2768–2801. https://doi.org/10.1093/eurheartj/ehw211
Dinoff A et al (2016) The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. PLoS One 11(9). https://doi.org/10.1371/JOURNAL.PONE.0163037
Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30(9):464–472. https://doi.org/10.1016/J.TINS.2007.06.011
Pialoux V, Brown AD, Leigh R, Friedenreich CM, Poulin MJ (2009) Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension 54(5):1014–1020. https://doi.org/10.1161/HYPERTENSIONAHA.109.138917
Salerno EA et al (2021) Physical activity patterns and relationships with cognitive function in patients with breast cancer before, during, and after chemotherapy in a prospective, nationwide study. J Clin Oncol 39(29):3283–3292. https://doi.org/10.1200/JCO.20.03514
Hiensch AE et al (2023) Moderators of exercise effects on self-reported cognitive functioning in cancer survivors: an individual participant data meta-analysis. J Cancer Survivorship 1–12. https://doi.org/10.1007/S11764-023-01392-3/FIGURES/1.
Jacobs W et al (2022) The effects of being informed about chemotherapy-related cognitive symptoms with and without self-affirmation on perceived cognitive symptoms of breast cancer patients: a randomized prospective, longitudinal study. Clin Breast Cancer 22(5):439–454. https://doi.org/10.1016/J.CLBC.2022.03.001
Lepage C et al. (2014) A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus 3(1). https://doi.org/10.1186/2193-1801-3-444
Billiet T et al (2018) Recovery from chemotherapy-induced white matter changes in young breast cancer survivors? Brain Imaging Behav 12(1):64–77. https://doi.org/10.1007/S11682-016-9665-8
Dranovsky A et al (2011) Experience dictates stem cell fate in the adult hippocampus. Neuron 70(5):908–923. https://doi.org/10.1016/J.NEURON.2011.05.022
Song J et al (2012) Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489(7414):150–154. https://doi.org/10.1038/NATURE11306
Lourenco F, Casey BJ (2013) Adjusting behavior to changing environmental demands with development. Neurosci Biobehav Rev 37(9):2233–2242. https://doi.org/10.1016/J.NEUBIOREV.2013.03.003
Takeuchi H et al (2010) Training of working memory impacts structural connectivity. J Neurosci 30(9):3297–3303. https://doi.org/10.1523/JNEUROSCI.4611-09.2010
Lövdén M et al (2010) Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia 48(13):3878–3883. https://doi.org/10.1016/J.NEUROPSYCHOLOGIA.2010.08.026
Ford I, Murray H, McCowan C, Packard CJ (2016) Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy 20-year follow-up of west of Scotland coronary prevention study. Circulation 133(11):1073–1080. https://doi.org/10.1161/CIRCULATIONAHA.115.019014/-/DC1
Wander GS, Bansal M (2018) Legacy effect in medicine—the expanding horizon! Indian Heart J 70(6):769. https://doi.org/10.1016/J.IHJ.2018.12.001
Llewellyn-Bennett R, Edwards D, Roberts N, Hainsworth AH, Bulbulia R, Bowman L (2018) Post-trial follow-up methodology in large randomised controlled trials: a systematic review. Trials 19(1). https://doi.org/10.1186/S13063-018-2653-0
Cutter DJ et al (2015) Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst 107(4). https://doi.org/10.1093/JNCI/DJV008
Van Nimwegen FA et al (2015) Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med 175(6):1007–1017. https://doi.org/10.1001/JAMAINTERNMED.2015.1180
Aleman BMP et al (2007) Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 109(5):1878–1886. https://doi.org/10.1182/BLOOD-2006-07-034405
Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C (2012) Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev 38(7):926–934. https://doi.org/10.1016/J.CTRV.2012.05.002
Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF (1999) Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85(3):640–50
Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B (2018) Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 52(3):154–160. https://doi.org/10.1136/BJSPORTS-2016-096587
Funding
This work was financially supported by the Dutch Cancer Society (KWF/Alpe, 10325/2016-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GS has received institutional research support from AstraZeneca, Merck, Novartis, Roche, and Seagen; and is a consultant for Biovica. Other authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Exercise intervention of PACT and PACES
Appendix: Exercise intervention of PACT and PACES
The moderate-to high-intensity exercise programs of the PACT and PACES studies comprised two sessions of aerobic and resistance exercise per week under the supervision of a trained physical therapist. The programs were tailored to the participants’ exercise capacity. The attendance rate was 81% and 73% for PACT and PACES, respectively [23, 24]. In addition, participants were encouraged to be physically active for at least 30 min per day on the remaining days of the week. Participants allocated to the low-intensity, home-based exercise program of PACES were coached by a trained oncology nurse and received written information on physical activity. PACT and PACES control participants were asked to maintain their pre-treatment physical activity levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naaktgeboren, W.R., Koevoets, E.W., Stuiver, M.M. et al. Effects of physical exercise during adjuvant chemotherapy for breast cancer on long-term tested and perceived cognition: results of a pragmatic follow-up study. Breast Cancer Res Treat 205, 75–86 (2024). https://doi.org/10.1007/s10549-023-07220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07220-7