Abstract
Purpose
An increased incidence of hypothyroidism among breast cancer survivors has been observed in earlier studies. The impact of the postoperative treatment modalities and their potential interplay on hypothyroidism development needs to be studied.
Methods
We conducted a population- and registry-based study using the Breast Cancer Data Base Sweden (BCBaSe) including females diagnosed with breast cancer between 2006 and 2012. In total, 21,268 female patients diagnosed with early breast cancer between 2006 and 2012, with no previous prescription of thyroid hormones and no malignant diagnosis during the last ten years before breast cancer diagnosis, were included in the final analysis.
Results
During the follow-up (median follow-up time 7.9 years), 1212 patients (5.7%) developed hypothyroidism at a median time of 3.45 years from the index date. No association of the systemic oncological treatment in terms of either chemotherapy or endocrine therapy and hypothyroidism development could be identified. A higher risk (HR 1.68;95% CI 1.42–1.99) of hypothyroidism identified among patients treated with radiation treatment of the regional lymph nodes whereas no increased risk in patients treated only with radiation therapy to the breast/chest wall was found (HR 1.01; 95% CI 0.86–1.19). The risk of hypothyroidism in the cohort treated with radiotherapy of the regional lymph nodes was present irrespective of the use of adjuvant chemotherapy treatment.
Conclusions
Based on the results of our study, the implementation of hypothyroidism surveillance among the breast cancer survivors treated with radiotherapy of the regional lymph nodes can be considered as reasonable in the follow-up program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer has become the leading malignant diagnosis since 2020 with more than two million new cases among women globally [1], while it is estimated that in 2040 about 3 million new cases will be diagnosed [2]. The rising incidence trend along with the reduced mortality over time among breast cancer patients result in an increased number of breast cancer survivors that is expected to rise in future. As a result, the understanding and recognition of long-term sequelae related to breast cancer diagnosis per se or treatment is essential to be able to adopt adequate strategies for prevention, early detection, and management of these conditions.
Hypothyroidism is relatively common in the general population with a prevalence of up to 5.3% with most of the cases being subclinical and consequently diagnosed as an accidental finding [3]. Although patients in the general population diagnosed with hypothyroidism seem to face an increased risk for multiple morbidities and higher mortality [4], hypothyroidism either at breast cancer diagnosis or diagnosed after breast cancer does not seem to affect cancer-specific or all-cause mortality [5].
In breast cancer patients, radiation therapy to the supraclavicular lymph nodes has been associated with increased risk for hypothyroidism [6, 7]. Some studies suggest a potential impact of systemic treatment to the risk of hypothyroidism in breast cancer patients as well [8,9,10,11,12,13,14]. However, the results from these studies are contradictory and inconclusive due to the small sample size [8, 10, 14], the lack of population-based data, and the lack of information on specific treatment approaches [10, 11, 13].
It is, therefore, essential to investigate the incidence and risk factors of hypothyroidism after breast cancer diagnosis in a population-based cohort including all relevant information on different treatment strategies, both systemic and locoregional.
In this population-based retrospective cohort study, we aimed to investigate the impact of different adjuvant treatment strategies on the risk for developing hypothyroidism after breast cancer diagnosis.
Methods
Study design, data source, and patient cohort
In this population- and register-based cohort study, we used the research database Breast Cancer Data Base Sweden (BCBaSe) as data source. BcBaSe is a database derived from the linkage of the National Quality Registry for Breast Cancer covering three healthcare regions in Sweden (Stockholm-Gotland, Uppsala-Örebro, North Region), which is corresponding to nearly 60% of Swedish population, with other national Registries of interest (the Prescribed Drug Registry, the National Patient Registry, and the Swedish cause of death registry).
Through BCBaSe, we identified all patients with non-metastatic breast cancer diagnosed at least one year after initiation of Prescribed Drug Registry (launched on July 1, 2005). Patients with bilateral breast cancer were also included. The date of breast cancer diagnosis was served as the index date.
We excluded men with breast cancer, patients with prior exposure to thyroid hormones (ATC-code: H03AA) before index date, and patients with prior cancer diagnosis (ICD-codes: C00-C14, C30-C32, C34, C50, C73) up to 10 years before index date.
Outcomes and definitions
The primary outcome was the frequency of new onset hypothyroidism in patients diagnosed with non-metastatic breast cancer. New onset hypothyroidism was defined as initiation of thyroid hormones (ATC-code: H03AA) from index date plus 90 days and onwards with at least two prescriptions irrespective of defined daily dose (DDD) without any prescription of antithyroid preparations (ATC-code: H03B) or surgical procedure to thyroid gland (ICD-10 codes: BAA40, BAA50, BAA60, and BAA99) at any time during the follow-up.
Secondary outcome was the identification of potential risk factors for development of hypothyroidism after breast cancer diagnosis with special interest in different oncological treatment strategies.
Data collection
The following data were extracted from the BCBaSe: age at diagnosis, date at breast cancer diagnosis, region, menopausal status, relevant autoimmune comorbidities that could be associated with increased risk for hypothyroidism and had a prevalence in the cohort enabling statistical analyses (diabetes mellitus type 1, seropositive or seronegative rheumatoid arthritis, systemic lupus erythematosus), any exposure to medications that can lead to hypothyroidism (lithium with ATC-code N05AN01; amiodarone with ATC-code C01BD01) after index date, T status, N status, histology, ER (estrogen receptor)-status, PgR (progesterone-receptor)- status, tumor grade, Her2-status (with IHC or FISH); type of primary surgery, oncological treatment including chemotherapy, radiotherapy, endocrine therapy, and anti-HER2 treatment.
In terms of oncological treatment, the retrieved information from BCBaSe corresponds to planned treatment. Due to a considerably high percentage of missing data regarding HER2 status and anti-HER2 treatment, we did not include this treatment strategy in our analyses. Regarding radiotherapy, the available information through BCBaSe was whether patients were planned for radiotherapy or not and the planned irradiated target as breast/chest wall, regional lymph nodes, or both. According to the Swedish guidelines during the study period, radiation therapy to regional lymph nodes included axillary stations level II to IV but not internal mammary nodal stations.
Statistical analysis
Summary statistics are presented as frequencies and percentages for categorical variables and medians and interquartile ranges for continuous variables. Time to diagnosis of hypothyroidism was analyzed using Cox proportional hazards models stratified on region, with breast cancer diagnosis date representing the index date. We allowed for competing risks by censoring in the event of emigration or death. Maximum follow-up date was 31 December 2018. Treatment variables were categorical and included: radiation therapy, chemotherapy and endocrine therapy. Separate univariate Cox models were fitted to each of these treatment variables to estimate unadjusted effects. A multivariable Cox model was used to estimate adjusted effects, which in addition to the above treatment variables included: exposure to amiodarone and lithium (any DDD) post index date; diagnoses of type 1 diabetes, rheumatoid arthritis with rheumatic factor, other rheumatoid arthritis, and systemic lupus erythematosus pre- or post- index date; and diagnosis date and age at diagnosis (continuous variables modeled as restricted cubic splines using three pre-specified knots). Effects were presented as Hazard Ratios (HR) and 95% Confidence Intervals (CI). The proportional hazards assumption was assessed using unique term and global Chi-squared hypothesis tests (α = 0.05) as well as plots of the Schoenfeld residuals. No violation of proportional hazards was detected, with the exception of amiodarone. We, therefore, fitted an additional multivariable model with the same parameterization as above, but also including an interaction between amiodarone and time to event, modeled as a restricted cubic spline with three pre-specified knots. We, then, computed cumulative incidence curves for radiotherapy (stratified on region) accounting for competing risks of emigration and death. All statistical analyses were performed in R studio version 2022.07.2, using R version 4.2.2 [15], relying heavily on the packages survival, tidycmprsk, and the tidyverse suite [16,17,18]
Results
Selection process and characteristics of patient cohort
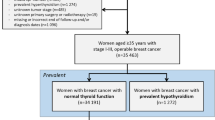
A flowchart of patient cohort selection process is illustrated in Fig. 1. Through the BCBaSe, 23,838 female patients with non-metastatic breast cancer were identified. After applying the exclusion criteria, 21,268 patients with breast cancer diagnosed between July 1st, 2006, and December 31st, 2012, were eligible for the study cohort (Fig. 1).
Patient-, tumor-, and treatment-related characteristics of patient cohort are presented in Table 1. The median age at diagnosis was 63 years (IQR 52–72 years). The percentage of patients treated with any adjuvant radiotherapy, endocrine therapy, and chemotherapy were 67.7%, 70.3%, and 31.8%, respectively. Regarding radiotherapy, the irradiated target included breast/chest wall only in 8953 patients (42.1%) whereas 5436 patients (25.6%) received radiotherapy to the regional lymph nodes as well.
Development of hypothyroidism after breast cancer diagnosis
During the follow-up (median follow-up 7.9 years; IQR 6.1–10.1), 1212 patients (5.7%) developed hypothyroidism with a median time of 3.45 years (IQR 1.67–5.65 years) from index date to diagnosis of hypothyroidism.
Impact of oncological treatment strategies on the risk for hypothyroidism
Table 2 presents the results from crude and adjusted analyses investigating the impact of different treatment strategies on the risk for hypothyroidism.
No association between use of endocrine therapy and development of hypothyroidism was observed, irrespective of the type of endocrine therapy (aromatase inhibitors, tamoxifen, or sequential therapy). In terms of chemotherapy use, a potential association between chemotherapy use (irrespective of the type of chemotherapeutic regimen) and hypothyroidism could be suspected in crude analyses but it was not confirmed in adjusted analyses.
Regarding radiotherapy, patients treated with radiotherapy including regional lymph nodes were at higher risk for developing hypothyroidism (HR 1.68; 95% CI 1.42–1.99) whereas no such association was observed in patients treated with radiotherapy only to the breast / chest wall (HR 1.01; 95% CI 0.86–1.19). In this adjusted analysis, patients treated with radiotherapy were stratified based on the use of adjuvant chemotherapy or not without resulting in any statistically significant interaction (p-value = 0.710).
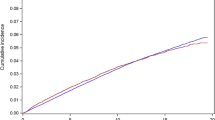
By analyzing the cumulative incidence of hypothyroidism stratified by radiotherapy use, cumulative incidence at 5 years for patients without radiotherapy, those treated with radiotherapy only to breast / chest wall, and those treated with radiotherapy to regional lymph nodes was 2.8% (95% CI 2.5–3.3%), 3.4% (95% CI 3.1–3.8%), and 6.1% (95% CI 5.5–6.8%), respectively, whereas the corresponding cumulative incidence at 10 years was 4.1% (95% CI 3.6–4.6%), 5.6% (95% CI 5.1–6.1%), and 8.7% (95% CI 7.9–9.5%), respectively (Fig. 2).
Discussion
In this population-based study, among breast cancer patients treated with multimodal approaches in the adjuvant setting, a higher risk for hypothyroidism was found only to those patients treated with radiotherapy including regional lymph nodes irrespective of the use of chemotherapy or not whereas no impact of systemic oncological treatment on hypothyroidism was observed. The considerably higher cumulative incidence of hypothyroidism in this patient group (8.7% in 10 years) supports the consideration of thyroid gland as organ-at-risk during treatment planning and the potential need for thyroid function screening as a part of follow-up strategy.
The higher risk for hypothyroidism in breast cancer patients treated with radiotherapy including regional lymph nodes is in accordance with previous studies [6, 7, 19]. The pathophysiological mechanism of this association, through direct thyroid cell injury or indirect due to damage in small thyroid vessels, has been described [20] and is further supported by dosimetric data showing higher mean radiation dose to the thyroid gland in patients treated with radiotherapy including regional lymph nodes compared to patients with radiotherapy only to breast / chest wall [19] and a dose-dependent association between dose to the thyroid gland and decreased thyroid volume [11]. The present study strengthens the current evidence by overcoming some of the main limitations of previous studies [21]. First, previous studies included a relatively small sample size [7, 22, 23], thus reducing their validity to provide reliable results on potential associations. Second, several studies lacked information on important risk factors for hypothyroidism, thus increasing the risk for confounding bias [7, 24]. Although we were not able to adjust for lifestyle factors as potential confounders, we could consider confounders related to comorbidities and other medications. Third, previous studies were prone to an upward biased estimation of incidence of hypothyroidism since they often did not account for competing events [25]. By applying suitable statistical approaches for competing events as emigration and death, our estimates might better reflect the expected incidence of hypothyroidism in this clinical situation.
An additional strength of the present study is the inclusion of systemic oncological treatment approaches in the analyses enabling the interrogation of the interplay and potential synergistic effect of systemic therapy and radiation therapy on thyroid dysfunction. Neither endocrine therapy nor chemotherapy were found to be associated with increased risk for hypothyroidism whereas radiotherapy including regional lymph nodes remained a risk factor when accounting for systemic treatment approaches.
In terms of endocrine therapy, a recent systematic review suggested a mild and transient thyroid dysfunction in patients treated with tamoxifen [8]. However, this transient thyroid dysfunction seems not to be translated into a clinical hypothyroidism that would need thyroid hormone replacement therapy according to our study results. In fact, our definition of hypothyroidism was solely based on the prescription of thyroid hormones after breast cancer diagnosis, thus reflecting only the occurrence of hypothyroidism that would be captured and treated.
Considering the potential impact of chemotherapy on the development of hypothyroidism, some preclinical evidence supports a potential association, mainly through altering the levels of thyroid hormone-binding proteins [26] or serving as radiosensitizer in the thyroid gland [27] does exist. However, the clinical evidence on this potential association is rather limited and uncertain with few studies suggesting some transient changes in thyroid hormone levels with questionable clinical relevance [28]. Our study results did not support either the potential direct impact of chemotherapy on hypothyroidism (no increased risk for hypothyroidism in chemotherapy-treated patients) or the hypothesis that chemotherapy might serve as radiosensitizer in the thyroid gland (similar magnitude of risk between patients treated with radiotherapy in regional lymph nodes with and without chemotherapy).
In our study cohort, the cumulative incidence of hypothyroidism at 10 years was found to be 8.7% in the cohort treated with radiotherapy including regional lymph nodes. It should be noted that this estimate is difficult to be compared with previous studies because of the various definitions used across the studies and the fact that we applied suitable statistical approaches to deal with competing events for calculation of cumulative incidence. Our estimate reflects the incidence of the occurrence of a clinical hypothyroidism required thyroid hormone replacement therapy but it does not consider overt hypothyroidism or hypothyroidism not requiring therapy. As a result, misclassification bias cannot be mitigated through our study design or method and should be considered as a potential limitation. However, one could argue that capturing hypothyroidism requiring therapy is a clinically relevant outcome with stronger clinical impact compared to subclinical hypothyroidism.
Apart from the above-mentioned limitations of misclassification bias and lack of information about lifestyle-related confounders, some additional limitations should be discussed. First, we lack volumetric and dosimetric data regarding radiotherapy. As a result, our results rely on the intended irradiated volume (breast/chest wall ± regional lymph nodes) and the translation of the national guidelines on target volume delineation during the study period. According to the guidelines, target volume for regional lymph nodes included level IV (supraclavicular area) using the caudal to the cricoid cartilage as cranial border. As radiation techniques are evolving and target volume delineation strategies are refined, 2015 ESTRO-guidelines recommended the subclavian artery arch for the cranial edge of level IV [29], thus reducing the mean dose to thyroid gland compared to the previous recommendation [19]. Our study results can, therefore, be applied to patients treated with radiotherapy to regional lymph nodes including level IV up to the cricoid cartilage whereas the potential impact of the 2015 ESTRO-recommendation on the risk of hypothyroidism should be further studied. An additional limitation is the fact that the treatment-related information captured in the BCBaSe refers to the planned treatment. Although some discrepancy between planned and given treatment can be anticipated, this proportion is expected to be low. Furthermore, we lack information on adherence to endocrine therapy among patients treated with this treatment strategy. However, two recent Swedish studies investigating the adherence to adjuvant endocrine therapy showed that more than 80% of the patients followed the prescribed medication 5 years after initiation [30, 31], thus supporting the notion that most of the patients included in our study cohort might also have followed the prescribed treatment. Finally, our study cohort lacks reliable information on trastuzumab use so this treatment strategy was not included into the analyses. Considering some case reports suggesting a potential association between trastuzumab and thyroid dysfunction [32], further studies including patients treated with anti-HER2 treatment strategies are required.
In conclusion, our study results confirm the increased risk of hypothyroidism in breast cancer patients treated with radiotherapy including regional lymph nodes as target volume. We found no association between systemic oncological treatment as endocrine therapy or chemotherapy and risk of hypothyroidism. Given the considerably high cumulative incidence of clinical hypothyroidism requiring thyroid hormone replacement therapy in the cohort of irradiated patients, it is motivated to incorporate thyroid gland as organ-at-risk during the treatment planning in order to gain detailed information on the radiation dose to the thyroid and evaluate dose–response associations. The high cumulative incidence also suggests a potential role of implementing regular monitoring of thyroid function as a part of the follow-up strategy in patients treated with radiotherapy including regional lymph nodes.
Data availability
The Breast Cancer DataBase Sweden (BCBaSe) cohort was used in this study. It is a population-based database that comprises all new cases of invasive breast cancer in women from 1992 to 2012 in three Swedish health care regions. The cohort was linked to a number of national population-based registries. Since BCBaSe contains sensitive health information, it cannot be published in open repositories. Those interested in data from BCBaSe should contact the corresponding author.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M et al (2022) Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66:15–23. https://doi.org/10.1016/j.breast.2022.08.010
Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM et al (2018) Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol 14(5):301–316. https://doi.org/10.1038/nrendo.2018.18
Sohn SY, Seo GH, Chung JH (2021) Risk of all-cause mortality in levothyroxine-treated hypothyroid patients: a Nationwide Korean Cohort Study. Front Endocrinol (Lausanne) 12:680647. https://doi.org/10.3389/fendo.2021.680647
McVicker L, Cardwell CR, McIntosh SA, McMenamin ÚC (2021) Cancer-specific mortality in breast cancer patients with hypothyroidism: a UK population-based study. Breast Cancer Res Treat 195(2):209–221. https://doi.org/10.1007/s10549-022-06674-5
Zhao XR, Fang H, Jing H, Tang Y, Song YW, Liu YP et al (2023) Radiation-induced hypothyroidism in patients with breast cancer after hypofractionated radiation therapy: a prospective cohort study. Int J Radiat Oncol Biol Phys 115(1):83–92. https://doi.org/10.1016/j.ijrobp.2022.04.052
Kanyilmaz G, Aktan M, Koc M, Demir H, Demir LS (2017) Radiation-induced hypothyroidism in patients with breast cancer: a retrospective analysis of 243 cases. Med Dosim 42(3):190–196. https://doi.org/10.1016/j.meddos.2017.03.003
Marina D, Rasmussen ÅK, Buch-Larsen K, Gillberg L, Andersson M, Schwarz P (2023) Influence of the anti-oestrogens tamoxifen and letrozole on thyroid function in women with early and advanced breast cancer: a systematic review. Cancer Med 12(2):967–982. https://doi.org/10.1002/cam4.4949
Huang J, Jin L, Ji G, Xing L, Xu C, Xiong X et al (2013) Implication from thyroid function decreasing during chemotherapy in breast cancer patients: chemosensitization role of triiodothyronine. BMC Cancer 13:334. https://doi.org/10.1186/1471-2407-13-334
Kumar N, Allen KA, Riccardi D, Bercu BB, Cantor A, Minton S et al (2004) Fatigue, weight gain, lethargy and amenorrhea in breast cancer patients on chemotherapy: is subclinical hypothyroidism the culprit? Breast Cancer Res Treat 83(2):149–159. https://doi.org/10.1023/b:brea.0000010708.99455.e1
Roberson J, Huang H, Noldner C, Hou W, Mani K, Valentine E et al (2022) Thyroid volume changes following adjuvant radiation therapy for breast cancer. Clin Transl Radiat Oncol 39:100566. https://doi.org/10.1016/j.ctro.2022.100566
Mazokopakis EE, Karefilakis CM, Tsartsalis AN, Milkas AN, Starakis IK (2008) Exemestane-induced subclinical hypothyroidism : a case report. Clin Drug Investig 28(10):669–671. https://doi.org/10.2165/00044011-200828100-00007
Reinertsen KV, Cvancarova M, Wist E, Bjøro T, Dahl AA, Danielsen T et al (2009) Thyroid function in women after multimodal treatment for breast cancer stage II/III: comparison with controls from a population sample. Int J Radiat Oncol Biol Phys 75(3):764–770. https://doi.org/10.1016/j.ijrobp.2008.11.037
Cutuli B, Quentin P, Rodier JF, Barakat P, Grob JC (2000) Severe hypothyroidism after chemotherapy and locoregional irradiation for breast cancer. Radiother Oncol 57(1):103–105. https://doi.org/10.1016/s0167-8140(00)00183-3
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Therneau T. A Package for Survival Analysis in R. R package version 3.4-0. https://CRAN.R-project.org/package=survival
Sjoberg D, Fei T. tidycmprsk: Competing Risks Estimation_. R package version 0.2.0. https://CRAN.R-project.org/package=tidycmprsk
Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R et al (2019) Welcome to the tidyverse. J Open Source Softwa 4(43):1686. https://doi.org/10.21105/joss.01686
Choi SH, Chang JS, Byun HK, Son NH, Hong CS, Hong N et al (2021) Risk of hypothyroidism in women after radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys 110(2):462–472. https://doi.org/10.1016/j.ijrobp.2020.12.047
Jereczek-Fossa BA, Alterio D, Jassem J, Gibelli B, Tradati N, Orecchia R (2004) Radiotherapy-induced thyroid disorders. Cancer Treat Rev 30(4):369–384. https://doi.org/10.1016/j.ctrv.2003.12.003
Solmunde E, Falstie-Jensen AM, Lorenzen EL, Ewertz M, Reinertsen KV, Dekkers OM et al (2023) Breast cancer, breast cancer-directed radiation therapy and risk of hypothyroidism: a systematic review and meta-analysis. Breast 68:216–224. https://doi.org/10.1016/j.breast.2023.02.008
Farshchian N, Amirifard N, Azar MHS, Heydarheydari S, Farshchian N, Haghparast A (2022) Thyroid function following radiation therapy in breast cancer patients: risk of radiation-induced hypothyroidism. Rep Pract Oncol Radiother 27(4):691–698. https://doi.org/10.5603/rpor.a2022.0074
Huang H, Roberson J, Hou W, Mani K, Valentine E, Ryu S et al (2021) NTCP model for hypothyroidism after supraclavicular-directed radiation therapy for breast cancer. Radiother Oncol 154:87–92. https://doi.org/10.1016/j.radonc.2020.09.003
Darvish L, Ghorbani M, Teshnizi SH, Roozbeh N, Seif F, Bayatiani MR et al (2018) Evaluation of thyroid gland as an organ at risk after breast cancer radiotherapy: a systematic review and meta-analysis. Clin Transl Oncol 20(11):1430–1438. https://doi.org/10.1007/s12094-018-1875-7
Coemans M, Verbeke G, Döhler B, Süsal C, Naesens M (2022) Bias by censoring for competing events in survival analysis. BMJ 378:e071349. https://doi.org/10.1136/bmj-2022-071349
Hamnvik OPR, Larsen PR, Marqusee E (2011) Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst 103(21):1572–1587. https://doi.org/10.1093/jnci/djr373
Hancock SL, Cox RS, McDougall IR (1991) Thyroid diseases after treatment of Hodgkin’s disease. N Engl J Med 325(9):599–605. https://doi.org/10.1056/nejm199108293250902
Mortezaee K, Ahmadi A, Haghi-Aminjan H, Khanlarkhani N, Salehi E, Shabani Nashtaei M et al (2019) Thyroid function following breast cancer chemotherapy: a systematic review. J Cell Biochem 120(8):12101–12107. https://doi.org/10.1002/jcb.28771
Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC, Biete Sola A et al (2015) ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 114(1):3–10. https://doi.org/10.1016/j.radonc.2014.11.030
Andersson A, Von Wachenfeldt VA, De Jong A, Nyström L (2019) Adherence to adjuvant endocrine therapy after breast cancer in Sweden 2008–2010: a nationwide survey. JCO 37(15_suppl):523–523
Lundgren C, Lindman H, Rolander B, Ekholm M (2018) Good adherence to adjuvant endocrine therapy in early breast cancer—a population-based study based on the Swedish Prescribed Drug Register. Acta Oncol 57(7):935–940. https://doi.org/10.1080/0284186x.2018.1442932
Sánchez-Bayona R, Garcia Del Barrio MA, Alegre E, Fernandez-Hidalgo OA, Eslava MS (2022) Trastuzumab and thyroid dysfunction: An association to be aware of. J Cancer Res Ther 18(4):1183–1185. https://doi.org/10.4103/jcrt.jcrt_66_19
Funding
Open access funding provided by Uppsala University. The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
ED contributed to methodology, formal analysis, investigation, and writing (original draft). DS contributed to methodology, formal analysis, and writing (review & editing). AM, ET, and AKW contributed to writing (review & editing). AV contributed to conceptualization, methodology, writing (original draft), and supervision.
Corresponding author
Ethics declarations
Competing interests
Financial interests: AM has performed consultancy for Veracyte (no financial or other compensation) and Roche (no financial or other compensation). ET has received speaker honoraria from Roche. Non-financial interests: AM has served on advisory boards for Veracyte and Roche, while he has received research funding paid to institution by AstraZeneca and Novartis. Authors ED, DS, AKW, and AV declare they have no conflict of interests.
Ethical approval
The study was approved by the Ethical Review Board in Stockholm (EPN Stockholm) dnr 2013/1272-31/4, with supplements approved dnr 2020-06312.
Consent to participate
Considering the observational nature of this register-based study, informed consent was waived from the Ethical Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Digkas, E., Smith, D.R., Wennstig, AK. et al. Incidence and risk factors of hypothyroidism after treatment for early breast cancer: a population-based cohort study. Breast Cancer Res Treat 204, 79–87 (2024). https://doi.org/10.1007/s10549-023-07184-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07184-8