Abstract
Purpose
Primary site surgery for metastatic breast cancer improves local control but does not impact overall survival. Whether histologic subtype influences patient selection for surgery is unknown. Given differences in surgical management between early-stage lobular versus ductal disease, we evaluated the impact of histology on primary site surgery in patients with metastatic breast cancer.
Methods
The National Cancer Database (NCDB, 2010–2016) was queried for patients with stage IV HR-positive, HER2-negative invasive lobular carcinoma (ILC) and invasive ductal carcinoma (IDC). We compared clinicopathologic features, primary site surgery rates, and outcomes by histologic subtype. Multivariable Cox proportional hazard models with and without propensity score matching were used for overall survival (OS) analyses.
Results
In 25,294 patients, primary site surgery was slightly but significantly less common in the 6,123 patients with ILC compared to the 19,171 patients with IDC (26.9% versus 28.8%, p = 0.004). Those with ILC were less likely to receive chemotherapy (41.3% versus 47.4%, p < 0.0001) or radiotherapy (29.1% versus 37.9%, p < 0.0001), and had shorter OS. While mastectomy rates were similar, those with ILC who underwent lumpectomy had significantly higher positive margin rates (ILC 15.7% versus IDC 11.2%, p = 0.025). In both groups, the odds of undergoing surgery decreased over time, and were higher in younger patients with T2/T3 tumors and higher nodal burden.
Conclusion
Lobular histology is associated with less primary site surgery, higher positive margin rates, less radiotherapy and chemotherapy, and shorter OS compared to those with HR-positive HER2-negative IDC. These findings support the need for ILC-specific data and treatment approaches in the setting of metastatic disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive lobular carcinoma (ILC) is the second most common histological subtype of breast cancer after invasive ductal carcinoma (IDC), representing 10–15% of all cases [1]. In the metastatic setting, ILC differs in its pattern of metastatic sites, often involving the bone, and gastrointestinal tract [2,3,4]. Additionally, several studies demonstrate worse overall survival (OS) in metastatic ILC compared to IDC, even when evaluating patients with similar receptor subtypes [4,5,6].
While investigators have evaluated surgical outcomes by histologic subtype in early-stage disease, there are scant data evaluating the use of primary-site surgery in the metastatic setting in those with ILC versus IDC. The current recommended therapy for metastatic breast cancer is systemic therapy, with local therapy reserved for palliation of symptoms [7]. While retrospective studies and institutional series have found associations between primary tumor resection and longer survival in those with metastatic breast cancer [8], most randomized control trials have not demonstrated such a survival advantage [9,10,11]. A previous study found that ILC patients with bone-only metastases had longer OS than those with visceral metastases when given a combination of chemotherapy and surgery, but it is unclear whether this reflects the more indolent course of osseous metastases, or an ILC specific effect of treatment [4, 12].
As such, whether histologic subtype should factor into patient selection for primary-site surgery is unknown. Prior analyses have suggested that if surgery of the primary tumor is associated with improved survival, this may be more likely in those with hormone receptor (HR) positive disease, or bone-only metastases [10, 13]. Given that ILC is largely HR-positive, HER2-negative, and has a propensity for bone metastases, we wondered if primary-site surgery use differed in patients with metastatic ILC compared to IDC.
We used the National Cancer Database (NCDB) to evaluate differences in practice patterns and management of patients with metastatic ILC compared to metastatic IDC. Specifically, we investigated the following questions: whether rates of primary-site surgery differ by histologic subtype and whether selection factors associated with undergoing primary-site surgery differ by histologic subtype. As secondary endpoints, we evaluated the use of chemotherapy and radiotherapy relative to surgery by histologic subtype, and the association between primary-site surgery and OS in ILC and IDC cohorts in both unmatched and propensity score matched multivariable models.
Methods
Data source and study cohort
The NCDB is a national comprehensive clinical surveillance resource representing over 70% of all newly diagnosed cancer cases in the United States and includes patient demographics, clinical information, and survival outcomes [14, 15]. Participants User Files from 2010 to 2016 were used. Due to the de-identified nature of the public-access user files, the study was exempted from institutional review board approval.
Since most ILC tumors are HR-positive and HER2-negative, we limited analysis to tumors with this receptor subtype. Tumors that were estrogen receptor (ER) and/or progesterone receptor (PR) positive were considered HR-positive. Histology codes were used to identify cohorts, with the ILC cohort comprising those with codes for ILC or mixed ILC/IDC (histology codes 8520, 8522, and 8524 if invasive behavior), and the IDC cohort comprising codes for IDC or invasive mammary carcinoma not otherwise specified (histology codes 8500, 8501, 8502, 8503, and 8523 if invasive behavior). We excluded patients with stage I-III disease, histologic subtypes other than IDC or ILC, individuals who died within 6 months of their diagnosis, and those missing critical clinical information including disease stage, HR-status, HER2-status, or treatment type.
Clinical measures
Charlson-Deyo Co-Morbidity Index (CDCI) was recorded as a measure of severity of co-morbid conditions. Age at diagnosis was subdivided into under 50 years and over 50 years to estimate pre- and post-menopausal status, respectively. Metastatic disease sites were categorized as bone-only, visceral-only, bone and visceral, or unknown [4]. We utilized data on treatment facility type, insurance status, and median income in univariate and multivariate analyses.
Statistical methods
We compared clinicopathologic and demographic features between the ILC and IDC cohorts using chi-square tests for categorical variables and t-tests for continuous variables. We investigated factors associated with receiving surgery for the primary tumor, radiotherapy, chemotherapy, and timing of chemotherapy relative to surgery by histologic subtype. For univariate analyses, we used Kaplan-Meier plots and log-rank tests to assess associations between receipt of surgery and OS by histologic subtype, and by timing of chemotherapy and receipt of radiotherapy. We also evaluated treatment facility type, insurance type, and median income quartiles by surgery and histologic subtype.
For multivariate analysis, we used Cox proportional hazards models to account for confounders with OS. The multivariable model included age, CDCI (0/1+), metastatic site (bone-only versus all other), and receptor subtype (ER-positive, PR-positive, HER2-negative versus ER-positive, PR-negative, HER2-negative).
Finally, we performed propensity score matching including age, tumor grade, receptor subtype, site of metastatic disease, CDCI (0/1+), and treating facility variables to account for likelihood of having primary-site surgery to determine the association between primary-site surgery and OS. Within each histology category among those who had survival data available patients who had surgery (ILC n = 1,444; IDC n = 4,924) were matched to patients who did not have surgery (ILC n = 3,553; IDC n = 10,894) using the greedy nearest neighbor matching algorithm. Matching was restricted to observations that had propensity scores in the extended common support region (ILC 0.05–0.71; IDC 0.06–0.66), which extends the common support region by 0.25 times a pooled estimate of the common standard deviation of the logit of the propensity score. The PSMATCH procedure in SAS version 9.4 was used to perform matching. To account for the matched nature of the sample, log-rank tests and Cox models were stratified on the matched pairs.
Hypothesis tests were two-sided, and the significance threshold was set to 0.05. Statistical analyses were performed using Stata 16 and SAS version 9.4.
Results
Study cohort
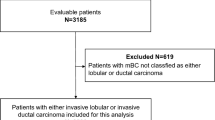
There were 100,147 patients with stage IV breast cancer, of whom 25,294 had HR-positive, HER2-negative invasive lobular or ductal histology, and met study criteria (Fig. 1). Of these patients, 19,171 (75.8%) had IDC and 6,123 (24.2%) had ILC. Within the ILC cohort, 4,484 (73.2%) had pure ILC, with the remaining having mixed ILC/IDC. Median follow-up time of the ILC cohort was 27.2 months (IQ range 14.7–41.5 months), which was similar to the median follow-up time of 26.8 months (IQ range 14.6–42.6 months) for the IDC cohort (Table 1). Patients with ILC were slightly older than those with IDC (mean age 64 years versus 61 years, p < 0.001) and differed significantly by race (p < 0.001), with a higher proportion of White patients (79.4% versus 74.2%, p < 0.0001) (Table 1). Additionally, ILC and IDC patients differed by tumor grade (p < 0.001), with ILC patients having a higher proportion of grade 1 tumors (22.1% versus 9.48%, p < 0.0001) and more bone-only metastases than those with IDC (60.8% versus 45.2%, p < 0.001).
CONSORT flow diagram for study population of stage IV, hormone receptor positive, HER2-negative breast cancer patients from the National Cancer Database (PUF 2010–2016). ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PUF, Participant User Files
While overall most patients were treated at community cancer centers, slightly more ILC patients than IDC patients were treated at academic centers (ILC 36.0% versus IDC 33.5%, p = 0.001) (Table 1). ILC patients were significantly more likely to have public insurance (ILC 54.7% versus IDC 51.6%) and less likely to be uninsured (ILC 3.82% versus IDC 5.15%, p < 0.0001). Patients with ILC had higher median income, with 39.7% in the highest quartile compared to 35.7% of IDC patients in the highest median income quartile (p < 0.0001).
Primary-site surgery by histologic subtype
In the overall study population, 7,158 (28.3%) underwent primary-site surgery. Although the absolute difference is small, primary-site surgery was performed less often in those with metastatic ILC than those with metastatic IDC (n = 1,644 [26.8%] versus n = 5,514 [28.8%] respectively, p = 0.004) (Table 2). This difference was more pronounced when restricting the analysis to patients with pure ILC, where only 23.8% underwent primary-site surgery. Additionally, the slightly lower rate of primary-site surgery among patients with ILC compared to IDC was observed among those with bone-only or unknown site of metastases, but not among those with visceral metastases (Fig. 2). Among those who had primary-site surgery in both histologic cohorts, the site of metastatic disease was significantly more likely to be bone-only compared to other sites (ILC 63.5%; IDC 48.5%, p < 0.001) (Table 3).
For both cohorts, patients with private insurance were significantly more likely to receive primary-site surgery (ILC 46.4%; IDC 49.4%, p < 0.0001) compared to patients with public insurance (Table 3). Both ILC and IDC patients who received surgery were equally likely to have been treated at an academic treating facility (ILC n = 415 [26.3%]; IDC n = 1,318 [26.2%]) compared to a community setting (ILC n = 1,164 [73.7%]; IDC n = 3,714 [73.8%]).
Among those who underwent primary-site surgery (n = 7,158), the differences by histology reflected those seen in the overall study population. Those with ILC were older (ILC mean age 61.7 years versus IDC 58.1 years, p < 0.001), had more T3 tumors (ILC 30.8% versus IDC 15.5%, p < 0.001), had more N3 nodal status (ILC 35.3% versus IDC 20.5%, p < 0.001), and had more grade 2 disease (ILC 61.4% versus IDC 45.9%, p < 0.001) (Table 1). There was no significant difference in mastectomy rate between the two cohorts (Table 2). Positive surgical margins were significantly more common in those with ILC who underwent lumpectomy compared to IDC (15.7% versus 11.2%, p = 0.025) (Table 1).
The factors associated with undergoing primary-site surgery were similar in the ILC and IDC cohorts. In both groups, primary-site surgery was less common in older patients, and more common in those with larger tumors (except T4) and higher N category (Table 4). The odds of undergoing primary-site surgery were highest for ILC patients with pathologic stage T3 disease versus T1 (OR 2.65, 95% CI 1.17–3.51, p = 0.002; Table 4) whereas the odds of surgery for patients with IDC were highest in pathologic stage T2 disease versus T1 (OR 1.71, 95% CI 1.30–2.25, p < 0.001). In both groups, those with T4 disease had significantly lower odds of primary-site surgery compared to T1 (Table 4). Over time, the odds of undergoing primary-site surgery decreased. Specifically, the odds of surgery decreased by 16% per each additional year of diagnosis (OR 0.84 and p < 0.001 for both groups; 95% CI 0.82–0.87 in ILC group; 95% CI 0.83–0.85 in IDC group, Table 4).
Radiotherapy by histologic subtype
The use of radiotherapy overall was lower for patients with ILC than IDC (29.1% versus 37.9%, p < 0.001) (Table 2). In IDC patients who had surgery, 51.5% also had radiation, while in ILC patients who had surgery, 42.5% also had radiation (Table 3). Among those who received radiotherapy, there was no difference in the rate of radiation to local versus distant sites by histologic subtype (p = 0.55).
Use and timing of chemotherapy
More IDC patients received chemotherapy (41.3% ILC versus 47.4% IDC, p < 0.001), while those with ILC were more likely to receive endocrine therapy (83.5% ILC versus 78.6% IDC, p < 0.001). For those who had primary-site surgery, the sequence of chemotherapy and surgery differed by histologic subtype; while 40.5% of patients with IDC had chemotherapy prior to surgery, only 29.0% of patients with ILC had chemotherapy prior to surgery (p < 0.001).
Survival analyses in unmatched cohorts
Overall, patients with ILC had slightly but significantly shorter OS than those with IDC (median 38 months ILC versus 40 months IDC, p = 0.006). In both cohorts, primary-site surgery was associated with significantly improved OS (Table 1). In the ILC cohort, undergoing primary-site surgery was associated with 35% lower risk of death compared to those who did not undergo surgery (HR 0.65, 95% CI 0.57–0.68, p < 0.001) (Fig. 3). This association persisted when controlling for age, CDCI (0/1+), metastatic site, and receptor subtype (HR 0.64, 95% CI 0.58–0.70, p < 0.001). The timing of surgery (before or after systemic chemotherapy) was not significantly associated with OS among those with ILC in unadjusted analysis nor after controlling for age, CDCI (0/1+), metastatic site, and receptor subtype.
Kaplan-Meier survival curves based on primary-site surgery and histology for stage IV hormone receptor positive, HER2-negative patients in the National Cancer Database (PUF 2010–2016). Estimated overall survival in patients with invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC) with or without surgery (unmatched, unadjusted cohorts)
Similarly, patients in the IDC cohort who underwent primary-site surgery had an associated 40% lower risk of death compared to those without surgery (HR 0.60, 95% CI 0.57–0.63, p < 0.001) (Fig. 3). This remained true after controlling for age, CDCI (0/1+), metastatic site, and receptor subtype (HR 0.61, 95% CI 0.58–0.64, p < 0.001). Unlike for those with ILC, timing of surgery was significantly associated with OS. Patients with IDC who had chemotherapy before surgery had 24% less risk of death compared to those who had surgery prior to chemotherapy (HR 0.76, 95% CI 0.70–0.84, p < 0.001). This association persisted after controlling for age, CDCI (0/1+), site of metastasis, and receptor subtype (HR 0.83, 95% CI 0.76–0.92, p < 0.001).
The type of surgery (mastectomy versus lumpectomy) was not significantly associated with different OS in either group. However, we found a significant statistical interaction between having surgery for the primary tumor and the site of radiation. Primary-site surgery was associated with a greater reduction in risk of death among those who had local radiation compared to those who had distant radiation (HR 0.35, 95% CI 0.3–0.4 versus HR 0.67, 95% CI 0.61–0.74 respectively, test of interaction p < 0.001). This interaction between primary-site surgery and site of radiation was similar in both the ILC and IDC cohorts separately.
Survival analyses in propensity score matched cohorts
The propensity score model included age, tumor grade, receptor subtype, metastatic site, CDCI (0/1+), and treating facility variables, with 3,089 ILC patients (991 with surgery, 2,098 without surgery) and 11,216 IDC patients (3,429 with surgery, 7,787 without surgery) in each cohort with available data for matching. In both the ILC and IDC matched samples there was still a significant association between having surgery for the primary tumor and improved OS (ILC HR 0.71, 95% CI 0.59–0.85, p < 0.001; IDC HR 0.67, 95% CI 0.61–0.74, p < 0.001).
Discussion
Recent randomized trial data suggest that the role of primary-site surgery in the management of patients with metastatic breast cancer is limited to local control in select cases, with no evidence of impact on OS [9,10,11]. However, optimal selection criteria for primary-site surgery are unknown, with decisions being made on an individualized basis in clinical practice [13]. Given the known differences in surgical management in the early stage setting and disease patterns in the metastatic setting between ILC and IDC, we explored whether the use of primary-site surgery differs in HR-positive HER2-negative metastatic lobular versus ductal breast cancer.
In this cohort of 25,294 patients from the NCDB, we found that a high proportion of patients overall underwent primary-site surgery in the setting of metastatic breast cancer (28.3%). It is important to note that these data represent patients diagnosed between 2010 and 2016, prior to the publication of randomized trials demonstrating the lack of OS benefit from primary-site surgery [9,10,11]. Indeed, we found that the odds of undergoing primary-site surgery significantly decreased over time. While primary-site surgery was more common in those with bone-only metastases, and those with ILC were more likely to have such a disease pattern, the overall usage of primary-site surgery in the ILC cohort was slightly but significantly lower than in the IDC cohort. Although a slightly smaller proportion of patients with ILC had primary-site surgery, the majority of factors associated with receiving surgery did not differ between the lobular and ductal groups; in both groups, primary-site surgery was more common among younger patients, those with T2 or T3 tumors, more nodal disease, and private insurance.
Interestingly, while patients with ILC had larger tumors than those with IDC, there was no difference in the rate of mastectomy by histologic subtype. This differs from the early-stage setting, where lobular histology is associated with higher mastectomy rates. Similar to the early-stage setting, however, those with metastatic ILC who had primary-site surgery experienced significantly higher positive margin rates after lumpectomy than those with metastatic IDC. This suggests that the local control benefit of primary-site surgery might be attenuated in those with ILC, who may require more extensive surgery to achieve negative margins. We did find an association between local radiotherapy and improved OS in this cohort; whether this association reflects a relationship between improved local control and survival outcomes versus improved outcomes in those selected to have radiation is unknown. Of note, patients with ILC were significantly less likely to receive radiation than those with IDC, which is consistent with other studies [16, 17].
Interestingly, we found significantly lower odds of primary-site surgery in patients with T4 tumors in both lobular and ductal groups. Since palliation is the most accepted purpose of primary-site surgery in the stage IV setting, we would have expected higher rates of surgery in those with T4 tumors. Alternatively, these tumors may have been deemed unresectable; one of the challenges of analyzing this retrospective dataset is the inability to discern the reasons for performing primary-site surgery.
This limitation likely impacts the strong association between primary-site surgery and improved OS that we found in both groups. For example, in both the ILC and IDC cohorts, patients who had private insurance were more likely to have surgery compared to patients who had public insurance. The improved outcomes associated with primary-site surgery may reflect improved access to care as opposed to a biologic effect of surgery. This is consistent with prior data; retrospective series tend to show a survival advantage associated with primary-site surgery [4, 12, 13], whereas recent randomized trial data do not [9, 11]. Such discrepancies suggest selection bias in which patients undergo primary-site surgery. We are likely unable to account for the many factors that influence why surgery would be used in some patients versus others despite using propensity score matched models.
Of more interest, perhaps, is the finding that the use of pre-operative systemic therapy was associated with improved OS in the IDC cohort, but not in the ILC cohort. We suspect that pre-operative systemic therapy in the IDC cohort may have helped to select patients who would have more durable response to therapy, and therefore have improved OS. In contrast, response to therapy in those with ILC may be more difficult to ascertain, or less likely to be associated with outcomes.
For systemic therapy, those with ILC were significantly more likely to receive endocrine therapy than those with IDC, despite all studied cases being HR-positive. Likewise, those with IDC were more likely to receive chemotherapy. This treatment pattern has been observed in previous literature and may point to the notion that early-stage ILC has reduced sensitivity to chemotherapies, or perceived as such, and is therefore utilized less frequently [18, 19]. However, more recent studies show that in the metastatic setting, response to eribulin and CDK4/6 inhibitors may be similar between ILC and IDC [20, 21]. These findings highlight the need to identify lobular specific therapies for those with metastatic disease.
As a secondary endpoint, we looked at OS by histology. Similar to our findings, worse OS in those with metastatic ILC has been shown in other studies as well [2, 3, 22]. While the underlying reason for this difference is unclear, it suggests that ILC is indeed biologically different than IDC, given differential outcomes despite restricting the study population to those with HR-positive, HER2-negative tumor types, and ILC tumors being of lower grade than IDC tumors. One potential explanation could be that those with metastatic ILC may have an overall higher burden of disease than is typically detected on standard imaging modalities [23].
To our knowledge, this is the largest reported study evaluating primary-site surgery by histologic subtype in metastatic breast cancer. However, this study is subject to several limitations, including selection bias, lack of detailed systemic therapy information, radiation field data, and the absence of local recurrence events as an endpoint. However, the findings reflect real-world management patterns which appear to differ by histologic subtype.
While ILC has long been regarded as a less aggressive tumor type, our findings from this large NCDB study are consistent with others showing worse outcomes in ILC than IDC. The differences between the IDC and ILC groups in this study were relatively small, however, it is interesting to note that histology appears to be influencing management. The use of primary-site surgery was slightly lower, and the use of both radiotherapy and chemotherapy were much lower in those with metastatic ILC compared to metastatic IDC. It is unclear what is driving the lower usage of chemotherapy and radiotherapy in ILC cases; this may reflect an underlying bias that lobular tumors are more indolent and slow growing. Coupled with shorter OS in the ILC cohort, these findings reinforce the need for further study to determine histologic subtype-specific management options. In regard to surgical management, the significantly larger tumor size and higher positive margin rates in the ILC cohort suggest that if primary-site surgery is to be utilized, one should consider a larger excision and likely incorporate radiotherapy to maximize potential benefit of locoregional intervention. Further work is needed to improve management outcomes for those with metastatic ILC.
Data Availability
The datasets analyzed during the current study are available in the National Cancer Database, https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/.
Abbreviations
- CDCI:
-
Charlson-Deyo Co-Morbidity Index
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormone receptor
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular carcinoma
- NCDB:
-
National Cancer Database
- OS:
-
Overall survival
- PR:
-
Progesterone receptor
References
Dossus L, Benusiglio PR (2015) Lobular breast cancer: incidence and genetic and non-genetic risk factors. Breast Cancer Res 17:37. https://doi.org/10.1186/s13058-015-0546-7
Mathew A, Rajagopal PS, Villgran V et al (2017) Distinct pattern of Metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd 77:660–666. https://doi.org/10.1055/s-0043-109374
Arpino G, Bardou VJ, Clark GM, Elledge RM (2004) Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 6:R149. https://doi.org/10.1186/bcr767
Sun MS, Yan HC, Gao M, Liu HJ, Xu L (2022) De novo metastatic lobular breast carcinoma: a population-based study from SEER database. Asian J Surg. https://doi.org/10.1016/j.asjsur.2021.12.036
Adachi Y, Ishiguro J, Kotani H et al (2016) Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer 16:248. https://doi.org/10.1186/s12885-016-2275-4
Orvieto E, Maiorano E, Bottiglieri L et al (2008) Clinicopathologic characteristics of invasive lobular carcinoma of the breast: results of an analysis of 530 cases from a single Institution. Cancer 113:1511–1520. https://doi.org/10.1002/cncr.23811
Khan SA (2007) Primary tumor resection in stage IV breast Cancer: consistent benefit, or consistent Bias? Ann Surg Oncol 14:3285–3287. https://doi.org/10.1245/s10434-007-9547-9
Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA (2007) Surgical removal of the primary tumor increases overall survival in patients with metastatic breast Cancer: analysis of the 1988–2003 SEER Data. Ann Surg Oncol 14:2187–2194. https://doi.org/10.1245/s10434-007-9438-0
Khan SA, Zhao F, Goldstein LJ et al (2022) Early local therapy for the primary site in De Novo Stage IV breast Cancer: results of a Randomized Clinical Trial (E2108). J Clin Oncol 40(9):978–987. https://doi.org/10.1200/JCO.21.02006
Soran A, Ozmen V, Ozbas S et al (2018) Randomized trial comparing resection of primary tumor with no surgery in stage IV breast Cancer at Presentation: protocol MF07-01. Ann Surg Oncol 25:3141–3149. https://doi.org/10.1245/s10434-018-6494-6
Badwe R, Hawaldar R, Nair N et al (2015) Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 16:1380–1388. https://doi.org/10.1016/S1470-2045(15)00135-7
Fields RC, Jeffe DB, Trinkaus K et al (2007) Surgical Resection of the primary tumor is Associated with increased long-term survival in patients with stage IV breast Cancer after Controlling for Site of Metastasis. Ann Surg Oncol 14:3345–3351. https://doi.org/10.1245/s10434-007-9527-0
Neuman HB, Morrogh M, Gonen M, Van Zee KJ, Morrow M, King TA (2010) Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer 116:1226–1233. https://doi.org/10.1002/cncr.24873
Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY (2009) Comparison of commission on Cancer–approved and –Nonapproved Hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol 27:4177–4181. https://doi.org/10.1200/JCO.2008.21.7018
Boffa DJ, Rosen JE, Mallin K et al (2017) Using the National Cancer Database for Outcomes Research: a review. JAMA Oncol 3:1722. https://doi.org/10.1001/jamaoncol.2016.6905
Luo Y, Ma A, Huang S, Yu Y (2021) Invasive lobular carcinoma has worse outcome compared with invasive ductal carcinoma in stage IV breast Cancer with bone-only metastasis. Breast Care 17:296–305. https://doi.org/10.1159/000521097
Yang C, Lei C, Zhang Y et al (2020) Comparison of overall survival between invasive lobular breast carcinoma and invasive ductal breast carcinoma: a propensity score matching study based on SEER Database. Front Oncol 10:590643. https://doi.org/10.3389/fonc.2020.590643
Mouabbi JA, Hassan A, Lim B, Hortobagyi GN, Tripathy D, Layman RM (2022) Invasive lobular carcinoma: an understudied emergent subtype of breast cancer. Breast Cancer Res Treat 193:253–264. https://doi.org/10.1007/s10549-022-06572-w
Barroso-Sousa R, Metzger-Filho O (2016) Differences between invasive lobular and invasive ductal carcinoma of the breast: results and therapeutic implications. Ther Adv Med Oncol 8:261–266. https://doi.org/10.1177/1758834016644156
Pérez-Garcia J, Cortés J, Metzger Filho O (2019) Efficacy of Single-Agent Chemotherapy for patients with Advanced Invasive Lobular Carcinoma: a pooled analysis from three clinical trials. Oncologist 24:1041–1047. https://doi.org/10.1634/theoncologist.2018-0182
Gao JJ, Cheng J, Bloomquist E et al (2020) CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol 21:250–260. https://doi.org/10.1016/S1470-2045(19)30804-6
Pestalozzi BC, Zahrieh D, Mallon E et al (2008) Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 international breast Cancer Study Group clinical trials. J Clin Oncol 26:3006–3014. https://doi.org/10.1200/JCO.2007.14.9336
Wilson N, Ironside A, Diana A, Oikonomidou O (2021) Lobular breast Cancer: a review. Front Oncol 10:591399. https://doi.org/10.3389/fonc.2020.591399
Funding
Author HTR was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) (Grant number TL1 TR 001871). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Author AMS is part of the Biostatistics Core that is generously supported by the University of California, San Francisco Department of Surgery. Author RAM is supported by National Cancer Institute Award K08CA256047. For the remaining authors no sources of funding were declared.
Author information
Authors and Affiliations
Contributions
Supervision, study concept and design, and interpretation of data were provided by Rita A Mukhtar. The first draft of the manuscript was written by Harriet Rothschild and all authors commented on previous versions of the manuscript. Amy Shui performed statistical analysis and data interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Due to the de-identified nature of the public-access user files in the National Cancer Database, the study was deemed exempt from institutional review board approval.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rothschild, H.T., Clelland, E.N., Abel, M.K. et al. The impact of histologic subtype on primary site surgery in the management of metastatic lobular versus ductal breast cancer: a population based study from the National Cancer Database (NCDB). Breast Cancer Res Treat 203, 245–256 (2024). https://doi.org/10.1007/s10549-023-07125-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07125-5