Abstract
Purpose
We aimed to assess the impact of surgery of primary tumor in overall survival (OS) of women with de novo metastatic breast cancer.
Methods
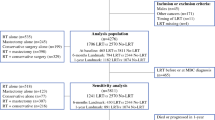
Nationwide, population-based retrospective cohort study of women diagnosed with de novo metastatic breast cancer in Belgium, between Jan/2010-Dec/2014. Data was obtained from the Belgian Cancer Registry and administrative databases. “Surgery” group was defined by surgery of primary tumor up to nine months after diagnosis. We excluded women who did not receive systemic treatment or did not complete nine months follow-up after diagnosis. All the subsequent analyses reporting on overall survival and the stratified outcome analyses were performed based on this nine-month landmark cohort. OS was estimated using Kaplan-Meier method and compared using adjusted Cox proportional hazards models controlling for confounders with 95% confidence intervals (CI). We performed a stratified analysis according to surgery timing and a propensity score matching analysis.
Results
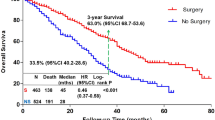
1985 patients, 534 (26.9%) in the “Surgery” and 1451 (73.1%) in the “No Surgery” group. Patients undergoing surgery were younger (p < 0.001), had better performance status (PS) (p < 0.001), and higher proportion of HER2-positive and triple-negative breast cancer (p = 0.012). Median follow-up was 86.0 months (82.6–88.5). Median OS was 60.1 months (57.1–68.2) in the “Surgery” vs. 41.9 months (39.8–44.2) in the “No Surgery” group (adjusted HR 0.56; 0.49–0.64). OS was similar when surgery was performed upfront or after systemic treatment. Propensity score matching analysis confirmed the same findings.
Conclusion
Among patients receiving systemic treatment for de novo metastatic breast cancer and surviving nine months or more, those who received surgery of the primary tumor within nine months of diagnosis have longer subsequent survival than those who did not.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the Belgian Cancer Registry but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Belgian Cancer Registry.
Change history
09 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10549-024-07275-0
Abbreviations
- CI:
-
Confidence interval
- HER2:
-
Human Epidermal growth factor Receptor type 2
- HR:
-
Hazard ratio
- ER:
-
Estrogen receptor
- ECOG:
-
Eastern Cooperative Oncology Group
- OS:
-
Overall survival
- PS:
-
Performance status
- TNBC:
-
Triple-negative breast cancer
References
Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA (2015) Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313(2):165–173
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33
Carmichael AR, Anderson EDC, Chetty U, Dixon JM (2003) Does local surgery have a role in the management of stage IV breast cancer? Eur J Surg Oncol 29(1):17–19
Petrelli F, Barni S (2012) Surgery of primary tumors in stage IV breast cancer: an updated meta-analysis of published studies with meta-regression. Med Oncol 29(5):3282–3290
Harris E, Barry M, Kell MR (2013) Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann Surg Oncol 20(9):2828–2834
Lu S, Wu J, Fang Y et al (2018) The impact of surgical excision of the primary tumor in stage IV breast cancer on survival: a meta-analysis. Oncotarget 9(14):11816
Soran A, Ozbas S, Kelsey SF, Gulluoglu BM (2009) Randomized trial comparing locoregional resection of primary tumor with no surgery in stage IV breast cancer at the presentation (protocol MF07-01): a study of turkish federation of the National Societies for breast Diseases. Breast J 15(4):399–403
Fitzal F, Bjelic-Radisic V, Knauer M et al (2019) Impact of breast surgery in primary metastasized breast Cancer: outcomes of the prospective Randomized Phase III ABCSG-28 POSYTIVE Trial. Ann Surg 269(6):1163–1169
Khan SA, Zhao F, Goldstein LJ et al (2022) Early local therapy for the primary site in De Novo Stage IV breast Cancer: results of a Randomized Clinical Trial (EA2108). J Clin Oncol 40(9):978–987
Badwe R, Hawaldar R, Nair N et al (2015) Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 16(13):1380–1388
Shien T, Hara F, Aogi K et al (2023) A randomized controlled trial comparing primary tumor resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (PRIM-BC): Japan Clinical Oncology Group study JCOG1017. 41(16suppl):523–523. https://doi.org/10.1200/JCO.2023.41.16_suppl.523
Soran A, Ozmen V, Ozbas S et al (2021) Primary surgery with systemic therapy in patients with de Novo Stage IV breast Cancer: 10-year Follow-up; protocol MF07-01 Randomized Clinical Trial. J Am Coll Surg 233(6):742–751e5
Tosello G, Torloni MR, Mota BS, Neeman T, Riera R (2018) Breast surgery for metastatic breast cancer. Cochrane Database Syst Rev 2018(3)
Tsukioki T, Shien T, Doihara H (2020) Effect of local surgery on outcomes of stage IV breast cancer. Transl Cancer Res 9(8):5102–5107
Cardoso F, Paluch-Shimon S, Senkus E et al (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol off J Eur Soc Med Oncol 31(12):1623–1649
Zeichner SB, Herna S, Mani A et al (2015) Survival of patients with de-novo metastatic breast cancer: analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Cancer Res Treat 153(3):617–624
Lane WO, Thomas SM, Blitzblau RC et al (2019) Surgical Resection of the primary tumor in Women with De Novo Stage IV breast Cancer: contemporary practice patterns and survival analysis. Ann Surg 269(3):537
Co M, Ng J, Kwong A (2019) De-novo metastatic breast cancers with or without primary tumor resection - A 10-year study. Cancer Treat Res Commun 19
Kim HJ, Kang E, Kim JH et al (2018) Survival Benefit of Surgical removal of primary tumor in patients with stage IV breast Cancer. Clin Breast Cancer 18(5):e1037–e1044
Pons-Tostivint E, Kirova Y, Lusque A et al (2019) Survival impact of Locoregional Treatment of the primary tumor in De Novo metastatic breast cancers in a large Multicentric Cohort Study: a propensity score-matched analysis. Ann Surg Oncol 26(2):356–365
Vohra NA, Brinkley J, Kachare S, Muzaffar M (2018) Primary tumor resection in metastatic breast cancer: a propensity-matched analysis, 1988–2011 SEER data base. Breast J 24(4):549–554
Wang K, Shi Y, Li ZY et al (2019) Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: a real-world observational study. Eur J Surg Oncol 45(8):1364–1372
Li X, Huang R, Ma L, Liu S, Zong X (2019) Locoregional surgical treatment improves the prognosis in primary metastatic breast cancer patients with a single distant metastasis except for brain metastasis. Breast 45:104–112
Wu SG, Zhang WW, Sun JY et al (2017) The survival benefits of local surgery in stage IV breast cancer are not affected by breast cancer subtypes: a population-based analysis. Oncotarget 8(40):67851–67860
Rosier L, Wang Y, Lee JH, Daily K (2022) Does definitive local therapy have a role in select HER2 + de novo metastatic breast cancer patients treated with dual anti-HER2 blockade? Breast Cancer Res Treat 191(2):375–383
Benchimol EI, Smeeth L, Guttmann A et al (2015) The REporting of studies conducted using Observational routinely-collected health data (RECORD) statement. PLoS Med 12(10)
Anderson JR, Cain KC, Gelber RD (2008) Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol 26(24):3913–3915
Giobbie-Hurder A, Gelber RD, Regan MM (2013) Challenges of guarantee-time bias. J Clin Oncol 31(23):2963–2969
van Walle L, Punie K, Van Eycken E et al (2021) Assessment of potential process quality indicators for systemic treatment of breast cancer in Belgium: a population-based study. ESMO Open 6(4):100207
Belgian Privacy Commission (2014) ‘Délibération n◦09/071 du 15 septembre 2009, modifiée le 18 février 2014, relative à la communication de données à caractère personnel par les organismes assureurs à la Fondation Registre du Cancer dans le cadre de l’article 45quinquies de l’AR n◦ 78 du 10’ https://www.privacycommission.be/sites/privacycommission/files/documents/délibération_SS_071_2009.pdf
Belgian Privacy Commission (2016) ‘Délibération N◦ 16/021 du 15 Mars 2016 relative à la communication de données à caractère personnel codées relatives à la santé par la cellule technique à la fondation registre du cancer pour l’estimation de la comorbidité chez les patients atteints de ca’ https://www.privacycommission.be/sites/privacycommission/files/documents/délibération_SS_071_2009.pdf
Soran A, Ozmen V, Ozbas S et al (2018) Randomized trial comparing resection of primary tumor with no surgery in stage IV breast Cancer at Presentation: protocol MF07-01. Ann Surg Oncol 25(11):3141–3149
Ruiterkamp J, Voogd AC, Tjan-Heijnen VCG et al (2012) Systemic therapy with or without up front surgery of the primary tumor in breast cancer patients with distant metastases at initial presentation. BMC Surg SUBMIT:12
Khan SA, Stewart AK, Morrow M (2002) Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 132(4):620–627
Kommalapati A, Tella SH, Goyal G, Ganti AK, Krishnamurthy J, Tandra PK (2018) A prognostic scoring model for survival after locoregional therapy in de novo stage IV breast cancer. Breast Cancer Res Treat 170(3):677–685
Thomas A, Khan SA, Chrischilles EA, Schroeder MC (2016) Initial surgery and survival in stage IV breast Cancer in the United States, 1988–2011. JAMA Surg 151(5):424–431
Mavrommati I, Johnson F, Echeverria GV, Natrajan R (2021) Subclonal heterogeneity and evolution in breast cancer. npj Breast Cancer 2021 71 7(1):1–9
Poggio F, Lambertini M, De Azambuja E (2018) Controversies in Oncology: surgery of the primary tumour in patients presenting with de novo metastatic breast cancer: to do or not to do? ESMO Open 3(1):e000324
Soran A, Dogan L, Isik A et al (2021) The Effect of primary surgery in patients with De Novo Stage IV breast Cancer with bone metastasis only (Protocol BOMET MF 14 – 01): a Multi-Center, prospective Registry Study. Ann Surg Oncol 28(9):5048–5057
Palma DA, Olson R, Harrow S et al (2020) Stereotactic ablative radiotherapy for the Comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 38(25):2830–2838
Chmura SJ, Winter KA, Woodward WA et al (2022) NRG-BR002: a phase IIR/III trial of standard of care systemic therapy with or without stereotactic body radiotherapy (SBRT) and/or surgical resection (SR) for newly oligometastatic breast cancer (NCT02364557). 40(16suppl):1007–1007. https://doi.org/10.1200/JCO.2022.40.16_suppl.1007
Hortobagyi GN, Stemmer SM, Burris HA et al (2022) Overall survival with Ribociclib plus Letrozole in Advanced breast Cancer. N Engl J Med 386(10):942–950
Swain SM, Baselga J, Kim S-B et al (2015) Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive metastatic breast Cancer. N Engl J Med 372(8):724–734
Verma S, Miles D, Gianni L et al (2012) Trastuzumab Emtansine for HER2-Positive advanced breast Cancer. N Engl J Med 367(19):1783–1791
Cortés J, Kim S-B, Chung W-P et al (2022) Trastuzumab Deruxtecan versus Trastuzumab Emtansine for breast Cancer. N Engl J Med 386(12):1143–1154
Modi S, Jacot W, Yamashita T et al (2022) Trastuzumab Deruxtecan in previously treated HER2-Low advanced breast Cancer. N Engl J Med
Bardia A, Hurvitz SA, Tolaney SM et al (2021) Sacituzumab Govitecan in Metastatic Triple-Negative breast Cancer. N Engl J Med 384(16):1529–1541
Rugo HS, Bardia A, Marmé F et al (2022) Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2-) advanced breast cancer. https://doi.org/10.1200/JCO.2022.40.17_suppl.LBA100140(17_suppl),LBA1001–LBA1001
Cortes J, Rugo HS, Cescon DW et al (2022) Pembrolizumab plus Chemotherapy in Advanced Triple-Negative breast Cancer. N Engl J Med 387(3):217–226
Aftimos P, Oliveira M, Irrthum A et al (2021) Genomic and transcriptomic analyses of breast Cancer primaries and matched Metastases in AURORA, the breast International Group (BIG) Molecular Screening Initiative. Cancer Discov 11(11):2796–2811
van Walle L, Vandeven J, Colpaert C et al (2020) Incidence of breast cancer subtypes in Belgium: a population-based study - BJMO. Belg J Med Oncol 14(6):263–273
Acknowledgements
We thank the Belgian Cancer Registry for the support provided and for the prior data collection of the immunohistochemistry subtypes for the women diagnosed with breast cancer in the year of 2014 included in this analysis [51].
Funding
Belgian Society of Medical Oncology (BSMO) supported the costs of access to Belgian Cancer Registry infrastructure, data coupling and training provided. The Fonds Pink Ribbon provided financial support for the manual retrieval of information from the pathology reports at the Belgian Cancer Registry.
Author information
Authors and Affiliations
Contributions
Conception/design: MB, CDA, NVD, EA. Provision of study material or patients: NVD, LvW. Collection and/or assembly of data: NVD, LvW. Data analysis and interpretation: MB, DMB, CDA, RDG, EA. Manuscript writing: MB, DMB. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing Interests
Research grants to Institut Jules Bordet (MB, DMB, CDA, AA, MP, EdA): from Roche/GNE, Radius, AstraZeneca, Lilly, MSD, GSJ/Novartis, Synthon, Servier, and Pfizer (all outside the submitted work). MB: travel grant from Roche, Takeda and Sanofi; speaker honoraria from Roche, Takeda and Janssen; advisory board from Sanofi (all outside the submitted work). DMB: honoraria from Daiichi Sankyo, Novartis, Merck Sharp & Dohme, Janssen, Pfizer, and Angelini; Meeting/travel grants from Novartis, Merck Sharp & Dohme, LEO Farmacêuticos, Ipsen, Janssen; (all outside the submitted work). PV: travel, speakers and advisory fees via Roche, MSD, BMS, Pfizer, Novartis, Lilly. ARF: honoraria and/or advisory board fees from Daiichi Sankyo, Gilead, Merck Sharp & Dohme, Novartis, Roche; meeting/travel grants: Roche (all outside the submitted work). ML: consultancy for Roche, Lilly, AstraZeneca, Pfizer, Novartis, MSD, Seagen, Exact Sciences, Gilead; speaker honoraria from Roche, Takeda, Ipsen, Sandoz, Knight, Libbs, Lilly, Pfizer, Novartis, Daiichi Sankyo, Gilead; travel grant from Gilead; institutional research grant from Gilead. FP: support for attending meetings from Daichii Sankyo and Gilead; participation on advisory board from AstraZeneca; and fees or honoraria from Eli Lilly and Novartis (all outside the submitted work). FPD: advisory role (support to Institution) for Amgen, AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead, Mundipharma, Novartis, Pfizer, Pierre Fabre, Roche, Seagen, and Teva. HW: his institution received financial compensation on his behalf for advisory boards, lecture fees and/or consultancy fees from Immutep Pty, MSD, Astrazenca, Daiichi, AbbVie, Lilly, Roche, EISAI, Pfizer, Sirtex, Gilead; travel grant from Pfizer and Roche. CC: employment at Breast International Group; institution research grant from AstraZeneca, Roche/Genentech, Tesaro, Novartis, Pfizer, SERVIER, Biovica, GlaxoSmithKline, and Sanofi/Aventis; institutional royalties from Agendia for MammaPrint; board member of European Society of Surgical Oncology (all outside of submitted work). AA: advisory role, research grants to my Institute, speaker fees: Roche, Lilly, Amgen, EISAI, BMS, Pfizer, Novartis, MSD, Genomic Health, Ipsen, AstraZeneca, Bayer, Leo Pharma. MPG: consultancy for AstraZeneca, Lilly, MSD, Novartis, Odonate, Pfizer, Roche, Camel-IDS, Crescendo Biologics, Periphagen, Huya, Debiopharm, PharmaMar, G1 Therapeutics, Menarini, Seattle Genetics, Immunomedics, and Oncoloytics; board member of Radius. KP: research grants to institute from MSD and Sanofi; speaker fees and honoraria for consultancy and advisory board functions from Astra Zeneca, Eli Lilly, Exact Sciences, Focus Patient, Gilead, MSD, Novartis, Pfizer, Roche, and Seagen; speaker fees and honoraria for consultancy and advisory board functions to institution from Astra Zeneca, Eli Lilly, Exact Sciences, Gilead, MSD, Novartis, Pfizer, Roche, and Seagen; stock options from Need Inc; travel grants from Astra Zeneca, Novartis, Pfizer, PharmaMar, and Roche. EdA: Honoraria and advisory board from Roche/GNE, Novartis and SeaGen. Travel grants from Roche/GNE and GSK/Novartis. Other authors: no conflicts of interest.
Ethics approval
Approval of the Ethical committee was not necessary based on the fact that this retrospective study does not fall under the Belgian Law of 7 May 2004 regarding experiments on human persons (art. 3,§ 2).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original publication of the article, the following article note has been missed to include. “Mariana Brandão and Diogo Martins-Branco have contributed equally to this work.”
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brandão, M., Martins-Branco, D., De Angelis, C. et al. Surgery of the primary tumor in patients with de novo metastatic breast cancer: a nationwide population-based retrospective cohort study in Belgium. Breast Cancer Res Treat 203, 351–363 (2024). https://doi.org/10.1007/s10549-023-07116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07116-6