Abstract
Purpose
Eribulin is a unique anti-cancer drug which can improve overall survival (OS) of patients with metastatic breast cancer (MBC), probably by modulating the tumor immune microenvironment. The aim of this study was to investigate the clinical significance of serum levels of immune-related and inflammatory cytokines in patients treated with eribulin. Furthermore, we investigated the association between cytokines and immune cells, such as myeloid-derived suppressor cells (MDSCs) and cytotoxic and regulatory T cells, to explore how these cytokines might affect the immune microenvironment.
Methods
Sixty-eight patients with MBC treated with eribulin were recruited for this retrospective study. The relationship of cytokines, including interleukin (IL)-6, to progression-free survival and OS was examined. CD4+ and CD8+ lymphocyte, MDSCs and regulatory T cell levels were determined in the blood by flow cytometry analysis.
Results
In our cohort, patients with high IL-6 at baseline had shorter progression-free survival and OS compared with those with low IL-6 (p = 0.0017 and p = 0.0012, respectively). Univariable and multivariable analyses revealed that baseline IL-6 was an independent prognostic factor for OS (p = 0.0058). Importantly, CD8+ lymphocytes were significantly lower and MDSCs were significantly higher in patients with high IL-6, compared to those with low IL-6.
Conclusion
Baseline IL-6 is an important prognostic factor in patients with MBC treated with eribulin. Our results show that high IL-6 is associated with higher levels of MDSCs which suppress anti-tumor immunity, such as CD8+ cells. It appears that eribulin is not particularly effective in patients with high IL-6 due to a poor tumor immune microenvironment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastatic and recurrent breast cancers tend to become refractory to chemotherapies, and a limited number of agents have been shown to further extend overall survival (OS) after treatment with major chemotherapeutic agents, such as taxanes and anthracyclines [1,2,3,4,5]. Eribulin mesylate, which is a unique inhibitor of microtubule dynamics, is one of the anti-cancer drugs that can extend the OS of patients with metastatic breast cancer (MBC) who have received at least two prior chemotherapy regimens for late-stage disease [6,7,8,9,10,11]. Interestingly, eribulin extends OS without extending progression-free survival (PFS) of patients with MBC [8]. This indicates that the effect of eribulin on the tumor microenvironment, such as suppression of epithelial-mesenchymal transition and improvement of the hypoxic microenvironment by vascular normalization, may affect treatment results after eribulin [12, 13]. Moreover, in the phase III EMBRACE trial, we identified that high absolute lymphocyte count (ALC) at baseline is significantly associated with longer OS in eribulin-treated patients, but not in patients treated with the physician’s choice, which strongly suggests an association between eribulin efficacy and immune response [14,15,16].
Although there is strong evidence that eribulin has a role in the tumor immune microenvironment to enhance anti-tumor immunity, the precise mechanisms involved in patients undergoing eribulin treatment are unknown. This is due to the difficulty of directly monitoring the tumor microenvironment in the daily clinical setting, which is currently only possible with repeated biopsies of the metastatic tumors. In addition, the ALC and neutrophil-to-lymphocyte ratio (NLR), which are related to the effects of eribulin [14, 15, 17], reflect the immune status of the whole body, and these parameters may not directly reflect the tumor immune microenvironment. Therefore, there is a paucity of data examining the actual tumor immune microenvironment in patients with MBC treated with eribulin.
Cytokines are involved in regulating the tumor microenvironment and can be measured in the blood [18, 19], potentially helping to monitor the tumor microenvironment in daily practice. Indeed, some cytokines are associated with the actions of eribulin, and may reflect changes in the tumor immune microenvironment. In this study, we focused on interleukin (IL)-6, soluble IL-2 receptor (sIL-2R) and tumor necrosis factor (TNF)-α as cytokines related to tumor immunity and microenvironment [20,21,22,23]. Furthermore, immunosuppressive immune cells, such as myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and cytotoxic CD8+ T cells play important roles to suppress anti-tumor immunity in the tumor microenvironment. Since MDSCs and Tregs are present both in the tumor microenvironment and in the blood, their role can be inferred from blood tests. The aim of this study was to investigate the clinical significance of serum levels of the immune and inflammatory cytokines, IL-6, sIL2-R and TNF-α, in patients with MBC treated with eribulin. Furthermore, we investigated the association between cytokines and immune cells, such as MDSCs and cytotoxic and regulatory T cells, in the blood of these patients to explore the effect of these cytokines on the immune microenvironment in patients treated with eribulin.
Materials and methods
Patient eligibility
A total of 68 patients with MBC treated with eribulin at Hyogo Medical University Hospital from December 2014 to March 2023 were recruited for this retrospective study. Patients were eligible if they had received more than one cycle of eribulin therapy. All participants were confirmed to have primary breast cancer through histologic examination, and a locally-advanced stage or metastasis was confirmed through diagnostic radiography using computed tomography, whole-body bone scintigraphy, or 2-([18]F)-fluoro-2-deoxy-D-glucose positron emission tomography.
All patients were classified by the combination of hormone receptor and human epidermal growth factor 2 (HER2) expression. Hormone receptor was considered negative if there were less than 1% positive tumor nuclei in the sample on immunohistochemical testing, in the presence of expected reactivity of internal (normal epithelial elements) and external controls. HER2-negative was defined as either an immunohistochemistry score of zero/1+ or 2+ with no HER2 amplification, as confirmed by in situ hybridization.
Determination of the NLR, ALC and cytokine levels in peripheral blood
Baseline values for ALC, NLR and cytokine levels, including IL-6, sIL-2R and TNF-α, were determined in peripheral blood before the day of the first treatment with eribulin. The neutrophil and lymphocyte counts were measured automatically using a Sysmex XN-9000 or XN-1000 hematology analyzer (Sysmex Corp, Kobe, Japan). NLR was defined as the neutrophil count divided by the lymphocyte count. Serum samples were sent to an external clinical laboratory (SLR, Inc. Tokyo, Japan) to determine IL-6, sIL-2R and TNF-α levels. IL-6 and sIL-2R were measured by chemiluminescent enzyme immunoassay (Fujirebio, Inc, Tokyo, Japan) and TNF-α was measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, US).
Flow cytometry analysis
For research purposes, we routinely perform flow cytometry analysis on fresh blood from patients undergoing chemotherapy if informed consent is obtained from the patient. Peripheral blood was collected in a tube and diluted with D-PBS (−) (Nacalai Tesque Inc., Kyoto, Japan) and layered onto Ficoll–Paque PLUS (Cytiva, Marlborough, US) using SepMate-50 tubes (STEM CELL Technologies, Vancouver, Canada). The tubes were centrifuged at 1200×g for 20 min at 20 °C. The top layer containing the enriched mononuclear cells was poured off and collected in another tube. The tubes were then centrifuged at 300×g for 10 min at 20 °C prior to washing. 1× RBC Lysis Buffer (Thermo Fisher Scientific, Waltham, US) was added to the cell pellet containing red blood cells, then the cells were washed twice with D-PBS (−). Cells were counted using a Countess 2 FL Automated Cell Counter (Thermo Fisher Scientific) and used for flow cytometry analysis.
Cells were incubated with human serum AB (GemCell, Seven Hills, Australia) for 30 min at 4 °C in the dark for blocking, then stained with conjugated monoclonal antibodies (mAbs) for 30 min at 4 °C in the dark. After staining, cells were washed with Cell Staining Buffer (BioLegend, San Diego, US), and then fixed with True-Nuclear 1× Fix Concentrate (BioLegend) for 15 min at room temperature in the dark for cell surface staining and for 45 min at room temperature for intracellular staining. After cell surface staining, cells for intracellular staining were stained with conjugated mAbs suspended in True-Nuclear 1× Perm Buffer (BioLegend) for 30 min. Stained cells were detected by a BD LSRFortessa X-20 cell analyzer (BD Biosciences, San Jose, US) and analyzed with BD FACSDiva software.
Immune phenotypic profiles
Tregs were defined as CD4+CD25+FoxP3+ cells, while MDSCs were defined as CD11b+CD14+CD33+ cells, according to previous studies [24,25,26]. The following immune cell subsets were analyzed using flow cytometry and the listed Abs. T-cell subsets: FITC-conjugated anti-CD4 mAb (RPA-T4, BioLegend); PE-conjugated anti-CD8α mAb (HT8a, BioLegend); and APC-conjugated with anti-CD3 mAb (HT3a, BioLegend). Tregs: Alexa Flour 488-conjugated anti-FoxP3 mAb (Clone: 259D, BioLegend); PE-conjugated anti-CD25 mAb (M-A251, BioLegend); and APC-conjugated anti-CD4 mAb (RPA-T4, BioLegend). MDSCs: FITC-conjugated with anti-CD14 mAb (63D3, BioLegend); PE-conjugated with anti-CD33 mAb (WM53, BioLegend); and APC-conjugated with anti-CD11b mAb (M1/70, BioLegend). Alexa Flour 488-conjugated with anti-mouse IgGk (MOPC-21, BioLegend) and PE-conjugated anti-mouse IgGk mAb (MOPC-21, BioLegend) were used for isotype controls.
Statistical analysis
ALC, NLR and each cytokine were classified into low and high groups, based on cut-off values for each factor, and the PFS and OS between low and high groups were compared by Kaplan–Meier plots. Univariable and multivariable analyses of clinicopathological factors contributing to OS prolongation were performed using the Cox proportional-hazards model to obtain hazard ratios and 95% confidence intervals. The relationships between the clinicopathologic characteristics and IL-6 were evaluated using the χ2 or Fisher’s exact test, as appropriate. p < 0.05 was considered to indicated a significant difference. All statistical analyses were performed using JMP Pro Version 15.
Results
Determination of optimal cut-off values for ALC, NLR, IL-6, sIL-2R and TNF-α for OS
The cut-off values for NLR and ALC were set at 3 and 1500/µL, respectively, in accordance with previous studies [14]. Based on the receiver operating characteristic curve calculated using the Youden index for area under the curve (AUC), the optimal cut-off values for OS were determined as 3.4 pg/mL for IL-6 (AUC, 0.734; sensitivity, 0.856; specificity, 0.621); 403 U/mL for sIL-2R (AUC, 0.731; sensitivity, 0.771; specificity, 0.634) and 0.73 pg/mL for TNF-α (AUC, 0.517; sensitivity, 0.289; specificity, 0.832).
PFS and OS of patients according to baseline levels of IL-6, sIL-2R and TNF-α
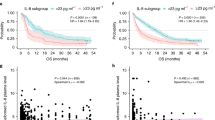
First, we assessed the PFS and OS of the 68 patients treated with eribulin according to baseline levels of IL-6, sIL-2R and TNF-α (Fig. 1). Patients with high baseline IL-6 levels showed significantly shorter PFS than those with low baseline IL-6 levels (p = 0.0017, Fig. 1A). Patients with high sIL-2R at baseline also showed significantly poorer PFS than those with low sIL-2R (p = 0.0394, Fig. 1B). Baseline TNF-α levels were not associated with PFS (p = 0.5405, Fig. 1C). Patients with high baseline IL-6 had poorer OS, compared with those with low IL-6 at baseline, and interestingly, the difference was even greater than with PFS (p = 0.0012, Fig. 1D). Patients with high sIL-2R at baseline also showed significantly shorter OS than those with low sIL-2R (p = 0.0219, Fig. 1E). Baseline TNF-α levels were not associated with OS (p = 0.2886, Fig. 1F).
Kaplan–Meier plots of progression-free survival (PFS) (A–C) and overall survival (OS) (D–F) in 68 patients treated with eribulin according to baseline levels of interleukin (IL)-6 (A, D), soluble IL-2 receptor (sIL-2R) (B, E) and tumor necrosis factor (TNF)-α (C, F). Blue and red lines show low and high levels, respectively
Univariable and multivariable analyses for OS in patients treated with eribulin
Next, we performed univariable and multivariable analyses for OS in patients treated with eribulin to assess the clinical impact of baseline IL-6, sIL-2R and TNF-α levels (Table 1). Univariable analysis revealed that IL-6 and sIL-2R levels were significant prognostic factors for OS (p = 0.0026 and p = 0.0258, respectively, Table 1). Multivariable analysis, including IL-6 and other clinical parameters, revealed that IL-6 level was an independent prognostic factor for OS (p = 0.0058, Table 1). However, multivariable analysis, including sIL-2R and other clinical parameters, revealed that sIL-2R level was not an independent prognostic factor for OS (p = 0.2827, Table 1).
Clinical impact of IL-6 levels at baseline and after treatment
Since IL-6 levels at baseline were significantly associated with the clinical outcomes of patients treated with eribulin, we next investigated which indicator was the most important among IL-6 levels: baseline, post-treatment, or changes in IL-6 between baseline and post-treatment. To this end, we assessed the PFS and OS of the 68 patients treated with eribulin according to their IL-6 levels after the first course of treatment with eribulin (Supplementary Fig. 1). Although post-treatment IL-6 levels were significantly associated with PFS (p = 0.0374, Supplementary Fig. 1A), post-treatment IL-6 levels were not significantly associated with OS (Supplementary Fig. 1B) in these patients. We next compared IL-6 levels at baseline and after the first treatment cycle in responders (PFS ≥ 12 months) and non-responders (PFS < 12 months) to assess the impact of changes in IL-6 before and after treatment. Most of the responders showed low IL-6 levels, both at baseline and at post-treatment (Fig. 2A). In contrast, most of the patients with high levels of IL-6, either at pre- or post-treatment, were non-responders (Fig. 2B).
Clinicopathological characteristics of patients with high IL-6 levels
Although our results indicate the importance of baseline IL-6 level as a prognostic indicator, we do not measure IL-6 levels in daily practice. Therefore, we examined the association of IL-6 level and other clinicopathological characteristics to reveal the characteristics of patients with high IL-6 levels at baseline. Univariable analysis revealed that patients with high baseline IL-6 had significantly lower albumin levels (p = 0.0007), higher C-reactive protein levels (p < 0.0001), higher modified Glasgow prognostic scores (p = 0.0064), lower prognostic nutritional indices (p = 0.0040), and lower platelet–lymphocyte ratios (p = 0.0361) than patients with low baseline IL-6 levels. Multivariable analysis revealed that patients with high baseline IL-6 had higher C-reactive protein levels (p = 0.0016), and lower prognostic nutritional indices (p = 0.0107) than patients with low baseline IL-6 levels.(Supplementary Table 1).
Anti-tumor immunity associated with IL-6 levels in patients treated with eribulin
We further explored the mechanisms underlying the poorer prognosis in patients with high IL-6 levels. We hypothesized that IL-6 levels might be associated with the state of anti-tumor immunity related to the treatment effect of eribulin, and so we analyzed the peripheral blood fractions for helper (CD4+) and cytotoxic (CD8+) lymphocytes, Tregs and MDSCs involved in tumor immunity at baseline. Interestingly, CD8+ lymphocytes, but not CD4+ lymphocytes, were significantly lower in patients with high IL-6, compared with those with low IL-6 (p = 0.0010 and p = 0.7972, respectively; Fig. 3A, B). Moreover, MDSCs were significantly higher in patients with high IL-6, compared with low IL-6 (p = 0.0190), although Tregs did not show any significant difference between the two patient groups (Fig. 3C, D).
Discussion
Eribulin is a distinctive anti-cancer drug which improves OS without extending PFS in patients with MBC [8]. ALC is a predictive marker for MBC patients treated with eribulin [14,15,16], and eribulin plays a role in regulating the tumor immune microenvironment [12, 13]; however, the underlying mechanisms of this regulatory role are not fully understood. In this study, we demonstrated that baseline IL-6 level is an independent prognostic factor for OS in patients with MBC treated with eribulin. Moreover, patients with high IL-6 levels had a low proportion of CD8+ cells and higher MDSC levels. These findings suggest the importance of an IL-6-associated immune mechanism underlying the effects of eribulin in patients with MBC.
Our current findings are in line with a previous study which showed that high IL-6 and IL-8 levels are significantly correlated with poorer survival of MBC patients treated with eribulin [27]. Transforming growth factor (TGF)-β is also reported to be an independent prognostic factor for MBC [28]. Basic research has shown that IL-6 and TGF-β closely interact with each other in the breast cancer microenvironment [29, 30], so both of these mediators may be associated with prognosis in patients receiving eribulin. Taken together, these results suggest that IL-6 is one of the major factors that predicts a poorer prognosis for patients with MBC treated with eribulin.
Our data also revealed that high IL-6 levels in MBC patients are associated with a low proportion of CD8+ lymphocytes, which play a central role in anti-tumor immunity [31, 32]. We also showed that high IL-6 was associated with high MDSC levels. It is well known that MDSCs suppress anti-tumor immunity, such as by inhibiting lymphocyte function, including CD8+ lymphocytes [33, 34]. Our findings indicate that a high level of IL-6 in MBC patients is associated with a pro-inflammatory tumor microenvironment, which promotes recruitment of MDSCs and suppresses anti-tumor immunity, including CD8+ lymphocyte activity.
In this study, baseline IL-6 levels reflected the clinical outcome of patients treated with eribulin, more so than post-treatment IL-6 levels, or changes in IL-6 levels before and after treatment. Others have also reported that baseline IL-6 levels are associated with the prognosis of patients treated with eribulin [27]. Furthermore, changes in TGF-β after treatment are also an important prognostic factor for MBC patients treated with eribulin [27]. It is possible that the difference in clinical significance of each cytokine reflects the difference in the role of each cytokine in the dynamic changes in tumor immunity. Moreover, in MBC patients, responders (non-progressive disease cases) to eribulin treatment have higher ALC at baseline, compared with non-responders, and the responders show further increases in ALC after treatment [35]. Taken together, these findings suggest that responders to eribulin have a favorable initial immune microenvironment, and that the immune microenvironment may be further improved with eribulin treatment. Considering that patients with low IL-6 had fewer MDSCs and higher CD8+ cells in our study, it is likely that a favorable immune microenvironment with low IL-6 level at baseline is especially important for the efficacy of eribulin.
Our data suggest that not only IL-6, but also immune cells, such as MDSCs and CD8+ cells, in peripheral blood are associated with the tumor microenvironment and patient outcome. Peripheral blood immune cell subsets, including NLR, have been reported to reflect the tumor microenvironment and anti-tumor immune response, and ultimately clinical outcome [36]. We have also reported that NLR and/or ALC reflect the outcome of breast cancer patients with some specific treatments [37,38,39]. These findings indicate that peripheral blood immune cells may reflect the tumor microenvironment of breast cancer patients, which is related to patient outcome.
This study has limitations. First, it was retrospective in nature with a comparatively small number of patients. Second, we did not measure other important cytokines, such as TGF-β. However, our data revealed important findings, including the clinical importance of IL-6 level at baseline as a prognostic marker for MBC patients treated with eribulin. Of note, as far as we know, this is the first study to reveal the involvement of MDSCs in the immune functions associated with IL-6 in eribulin treatment. Therefore, we believe that our data are valuable for understanding the actions of eribulin in the tumor immune microenvironment.
In conclusion, we suggest that the baseline IL-6 level is an important prognostic factor in patients with MBC treated with eribulin. Since high IL-6 induces MDSCs and suppresses anti-tumor immunity, reflected by reduced CD8+ lymphocyte counts, it is possible that eribulin is not sufficiently effective in patients with high IL-6 levels due to a poor tumor immune microenvironment.
Data availability
The data are not publicly available, and will be shared on reasonable request to the corresponding author.
References
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN (2007) Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol 608:1–22
Colozza M, de Azambuja E, Personeni N, Lebrun F, Piccart MJ, Cardoso F (2007) Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist 12(3):253–270
Dean-Colomb W, Esteva FJ (2008) Emerging agents in the treatment of anthracycline- and taxane-refractory metastatic breast cancer. Semin Oncol 35(Suppl 2):S31–S38
Andreopoulou E, Sparano JA (2013) Chemotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer: an overview. Curr Breast Cancer Rep 5(1):42–50
Jerusalem G, Rorive A, Collignon J (2015) Chemotherapy options for patients suffering from heavily pretreated metastatic breast cancer. Future Oncol 11(12):1775–1789
Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsky BM, Palme MH, Habgood GJ, Singer LA, Dipietro LV, Wang Y, Chen JJ, Quincy DA, Davis A, Yoshimatsu K, Kishi Y, Yu MJ, Littlefield BA (2001) In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin b. Cancer Res 61(3):1013–1021
Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, Littlefield BA, Wilson L (2005) The primary antimitotic mechanism of action of the synthetic halichondrin e7389 is suppression of microtubule growth. Mol Cancer Ther 4(7):1086–1095
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377(9769):914–923
Gourmelon C, Frenel JS, Campone M (2011) Eribulin mesylate for the treatment of late-stage breast cancer. Expert Opin Pharmacother 12(18):2883–2890
O’Sullivan Coyne G, Walsh J, Kelly CM (2012) Effectiveness and safety of eribulin mesylate: a new therapeutic option in the treatment of metastatic breast cancer. Expert Opin Drug Saf 11(4):643–650
Nakamoto S, Watanabe J, Ohtani S, Morita S, Ikeda M (2022) Eribulin improved the overall survival from the initiation of first-line chemotherapy for her2-negative advanced breast cancer: a multicenter retrospective study. BMC Cancer 22(1):31
Ueda S, Saeki T, Takeuchi H, Shigekawa T, Yamane T, Kuji I, Osaki A (2016) In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br J Cancer 114(11):1212–1218
Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, Tohyama O, Uehara T, Kimura T, Watanabe H, Asano M, Kawano S, Tizon X, McCracken PJ, Matsui J, Aoshima K, Nomoto K, Oda Y (2014) Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci 105(10):1334–1342
Miyoshi Y, Yoshimura Y, Saito K, Muramoto K, Sugawara M, Alexis K, Nomoto K, Nakamura S, Saeki T, Watanabe J, Perez-Garcia JM, Cortes J (2020) High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin-but not with treatment of physician’s choice-in the embrace study. Breast Cancer 27(4):706–715
Takahashi M, Inoue K, Mukai H, Yamanaka T, Egawa C, Miyoshi Y, Sakata Y, Muramoto K, Ikezawa H, Matsuoka T, Tsurutani J (2021) Indices of peripheral leukocytes predict longer overall survival in breast cancer patients on eribulin in japan. Breast Cancer 28(4):945–955
Goto W, Kashiwagi S, Asano Y, Takada K, Morisaki T, Fujita H, Takashima T, Ohsawa M, Hirakawa K, Ohira M (2018) Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res 38(5):2929–2938
Miyagawa Y, Araki K, Bun A, Ozawa H, Fujimoto Y, Higuchi T, Nishimukai A, Kira A, Imamura M, Takatsuka Y, Miyoshi Y (2018) Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer 18(5):400–409
Paccagnella M, Abbona A, Michelotti A, Geuna E, Ruatta F, Landucci E, Denaro N, Vanella P, Lo Nigro C, Galizia D, Merlano M, Garrone O (2022) Circulating cytokines in metastatic breast cancer patients select different prognostic groups and patients who might benefit from treatment beyond progression. Vaccines 10(1):78
Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, Delort L (2023) Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int J Mol Sci 24(4):4002
Dethlefsen C, Højfeldt G, Hojman P (2013) The role of intratumoral and systemic il-6 in breast cancer. Breast Cancer Res Treat 138(3):657–664
Wang L, Miyahira AK, Simons DL, Lu X, Chang AY, Wang C, Suni MA, Maino VC, Dirbas FM, Yim J, Waisman J, Lee PP (2017) IL6 signaling in peripheral blood T cells predicts clinical outcome in breast cancer. Cancer Res 77(5):1119–1126
Murakami S (2004) Soluble interleukin-2 receptor in cancer. Front Biosci 9:3085–3090
Yang Y, Lundqvist A (2020) Immunomodulatory effects of IL-2 and IL-15; implications for cancer immunotherapy. Cancers 12(12):3586
Zhang M, Berndt BE, Chen JJ, Kao JY (2008) Expression of a soluble tgf-beta receptor by tumor cells enhances dendritic cell/tumor fusion vaccine efficacy. J Immunol 181(5):3690–3697
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC (2005) Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65(8):3044–3048
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kübler H, Yancey D, Dahm P, Vieweg J (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 14(24):8270–8278
Garrone O, Michelotti A, Paccagnella M, Montemurro F, Vandone AM, Abbona A, Geuna E, Vanella P, De Angelis C, Lo Nigro C, Falletta A, Crosetto N, Di Maio M, Merlano M (2020) Exploratory analysis of circulating cytokines in patients with metastatic breast cancer treated with eribulin: the transeri-gono (gruppo oncologico del nord ovest) study. ESMO Open 5(5):e000876
Kashiwagi S, Asano Y, Goto W, Takada K, Morisaki T, Kouhashi R, Yabumoto A, Tanaka S, Takashima T, Ohsawa M, Hirakawa K, Ohira M (2020) Validation of systemic and local tumour immune response to eribulin chemotherapy in the treatment of breast cancer. Anticancer Res 40(6):3345–3354
Chin AR, Wang SE (2014) Cytokines driving breast cancer stemness. Mol Cell Endocrinol 382(1):598–602
Teng X, Hayashida T, Murata T, Nagayama A, Seki T, Takahashi M, Kitagawa Y (2021) A transposon screen identifies enhancement of NF-κB pathway as a mechanism of resistance to eribulin. Breast Cancer 28(4):884–895
Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM (2000) Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity 13(2):265–276
Klein L, Trautman L, Psarras S, Schnell S, Siermann A, Liblau R, von Boehmer H, Khazaie K (2003) Visualizing the course of antigen-specific cd8 and cd4 t cell responses to a growing tumor. Eur J Immunol 33(3):806–814
Palazón-Carrión N, Jiménez-Cortegana C, Sánchez-León ML, Henao-Carrasco F, Nogales-Fernández E, Chiesa M, Caballero R, Rojo F, Nieto-García MA, Sánchez-Margalet V, de la Cruz-Merino L (2021) Circulating immune biomarkers in peripheral blood correlate with clinical outcomes in advanced breast cancer. Sci Rep 11(1):14426
Zalfa C, Paust S (2021) Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Front Immunol 12:633205
Goto W, Kashiwagi S, Takada K, Asano Y, Morisaki T, Shibutani M, Tanaka H, Hirakawa K, Ohira M (2022) Utility of follow-up with absolute lymphocyte count in patients undergoing eribulin treatment for early detection of progressive advanced or metastatic breast cancer. Anticancer Res 42(2):939–946
Hwang M, Canzoniero JV, Rosner S, Zhang G, White JR, Belcaid Z, Cherry C, Balan A, Pereira G, Curry A, Niknafs N, Zhang J, Smith KN, Sivapalan L, Chaft JE, Reuss JE, Marrone K, Murray JC, Li QK, Lam V, Levy BP, Hann C, Velculescu VE, Brahmer JR, Forde PM, Seiwert T, Anagnostou V (2022) Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer 10(6):e004688
Imamura M, Morimoto T, Egawa C, Fukui R, Bun A, Ozawa H, Miyagawa Y, Fujimoto Y, Higuchi T, Miyoshi Y (2019) Significance of baseline neutrophil-to-lymphocyte ratio for progression-free survival of patients with her2-positive breast cancer treated with trastuzumab emtansine. Sci Rep 9(1):1811
Miyagawa Y, Yanai A, Yanagawa T, Inatome J, Egawa C, Nishimukai A, Takamoto K, Morimoto T, Kikawa Y, Suwa H, Taji T, Yamaguchi A, Okada Y, Sata A, Fukui R, Bun A, Ozawa H, Higuchi T, Fujimoto Y, Imamura M, Miyoshi Y (2020) Baseline neutrophil-to-lymphocyte ratio and c-reactive protein predict efficacy of treatment with bevacizumab plus paclitaxel for locally advanced or metastatic breast cancer. Oncotarget 11(1):86–98
Kanaoka H, Nagahashi M, Atake Y, Hattori A, Bun A, Fukui R, Ozawa H, Fujimoto Y, Higuchi T, Natori K, Imamura M, Murase K, Takatsuka Y, Miyoshi Y (2022) Absolute lymphocyte count is an independent prognostic factor for er-positive her2-negative advanced breast cancer patients treated with cdk4/6 inhibitors. Anticancer Res 42(10):4867–4878
Acknowledgements
We thank ClearScience (http://www.clearscience.net/) for English language editing.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research Grant Number 22H03140 and 21K19522 for MN, and 22K08764 for YM.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. AB, MN and YM contributed to data collection and statistical analysis. M Kuroiwa and M Komatsu contributed to flowcytometry analysis. YM supervised the entire study. AB and MN wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MN received honoraria from Chugai, AstraZeneca, Eli Lilly, Pfizer, Novartis, Taiho, Daiichi Sankyo, Esai, Kyowa-Kirin and Denka. YM received research funding and honoraria from Esai, Chugai, AstraZeneca, Eli Lilly, Pfizer, MSD, Kyowa-Kirin, Daiichi-Sankyo and Taiho. AB, M Kuroiwa and M Komatsu have no conflicts of interest to declare.
Ethical approval
This study was approved by the Ethics Committee of the Hyogo College of Medicine (No. 106) and was conducted following the Declaration of Helsinki. Written informed consent was obtained from all patients whose samples were used for measurement of cytokines and flow cytometry analysis.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bun, A., Nagahashi, M., Kuroiwa, M. et al. Baseline interleukin-6 is a prognostic factor for patients with metastatic breast cancer treated with eribulin. Breast Cancer Res Treat 202, 575–583 (2023). https://doi.org/10.1007/s10549-023-07086-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07086-9