Abstract
Purpose
Recent guidelines defined a new reporting category of ER-low-positive breast cancer based on immunohistochemistry (IHC). While low positivity of either hormone receptor is uncommon in invasive lobular carcinoma (ILC), we sought to investigate whether relatively low hormone receptor positivity was associated with tumor characteristics and patient outcomes in a single institutional cohort.
Methods
We searched an institutional database for cases of stage I-III ILC with available IHC reports. Based on prior published categories in ILC, ER was classified as low, medium, or high as defined by ER staining of 10–69%, 70–89%, and ≥ 90% respectively. PR low and high tumors were defined by < 20%, or ≥ 20% staining respectively. We used chi-squared tests, t-tests, and Cox proportional hazards models to evaluate associations between ER/PR categories and tumor characteristics or disease-free survival (DFS).
Results
The cohort consisted of 707 ILC cases, with 11% of cases categorized as ER low, 15.1% as medium, and 73.8% as high. The majority (67.6%) were PR high. Patients with ER low/medium expression were significantly younger, and more likely to also have PR low and/or HER2 positive tumors compared to those that were ER high. In a Cox proportional hazards model adjusting for age, stage, grade, pleomorphic histology, and treatment, ER category was not prognostic for DFS, but PR negative and PR low status each had significantly worse DFS compared to PR high status (HR 3.5, 95% CI 1.8–6.7, p < 0.001; and HR 2.0, 95% CI 1.1–3.5, p = 0.015, respectively).
Conclusion
These findings highlight the relevance of quantifying ER and PR within ILC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hormone receptor status in breast cancer is an important predictive marker for treatment response and outcomes. Current guidelines recommend endocrine therapy in those with ≥ 1% estrogen receptor (ER) positivity by immunohistochemistry (IHC). However, the degree of ER positivity has been thought to imply differential sensitivity to endocrine therapy [1]. This recognition has led to a recent introduction of a new reporting category for “ER-low positive” breast cancer from the American Society of Clinical Oncology and College of American Pathologists, defined as tumors having 1–10% ER expression by IHC [2].
Subsequently, investigators have evaluated the clinical implications of such ER-low positive status, with some analyses showing no difference between ER-low and ER strongly positive tumors, and others showing that ER-low positive tumors are more similar to ER-negative tumors in regard to outcomes [3,4,5,6]. However, very little data exist evaluating the spectrum of ER positivity in the setting of patients with invasive lobular carcinoma (ILC), the second most common type of breast cancer [7].
ILC is known to be a hormonally driven tumor type, with studies showing that combined estrogen and progestin hormone therapy confers an increased predisposition to ILC specifically [8]. Prior studies show high rates of strong ER positivity in most ILC tumors; indeed strictly ER-low status (1–10% positive) is very rare in ILC, with most cases being ≥ 90% ER-positive [3]. However, there remains a range of ER positivity within ILC, yet very little data evaluating heterogeneity within ILC based on level of ER expression.
In this study, we evaluated an institutional cohort of patients with stage I-III ILC to determine whether relatively low, intermediate, or high ER positivity defined clinically distinct subsets of tumors. Additionally, we evaluated the impact of progesterone receptor (PR) expression in conjunction with ER status. We hypothesized that even within highly ER-positive ILC cases, relatively lower ER and/or PR may be associated with distinct tumor features, treatment patterns, response to therapy, and clinical outcomes.
Methods
With approval from the institutional review board (#22-37379), we abstracted clinicopathologic data from a prospectively maintained institutional database containing treatment and outcomes data for ILC patients undergoing surgery at our institution between January 1996 and September 2019.
Population
We included patients with tumors that had lobular or mixed lobular/ductal histology and were diagnosed with stage I–III disease. Based on prior reported categories in ILC, we classified ER as relatively low, medium, or high expression as defined by ER staining of 10–69%, 70–89%, and ≥ 90% respectively [8]. Those with tumors ER < 10% were excluded from the analysis (Fig. 1). PR low and high tumors were defined by < 20%, or ≥ 20% staining respectively, as previously described in the literature [9]. Additionally, we evaluated combinations of ER and PR. These combined categories were classified as ER/PR low (both receptor categories low), ER/PR intermediate (one receptor category high or medium), or ER/PR high (both receptor categories high).
Clinicopathological parameters
The following clinicopathological parameters were evaluated by ER category and PR category individually, and also in combined ER/PR categories: age at diagnosis, body mass index (BMI), tumor stage, tumor histologic grade, human epidermal growth factor receptor-2 (HER2) overexpression status, treatment (local and systemic), and recurrence outcomes. HER2 positivity was defined by 3 + staining on IHC or positive in situ hybridization and Ki-67 was defined as high if greater than 14 percent staining was present.
Statistical analysis
We used chi-squared tests, t-tests, and Cox proportional hazards models in Stata 16.1 to evaluate associations between ER/PR categories (individually and in combination) with clinicopathologic variables, treatment, and surgical outcomes. We evaluated the association between individual ER and PR categories with disease-free survival (DFS) in multivariable models adjusting for patient age at diagnosis, tumor grade, stage, treatment, and HER2 status [10]Finally, we performed a test of interaction between ER category and receipt of adjuvant endocrine therapy to predict DFS. DFS was defined as the time from the date of diagnosis to the date of local recurrence, distant recurrence, or death; patients alive without disease recurrence were censored at the date of last follow-up. We used the log-rank test and Kaplan Meier method, and multivariate Cox proportional hazards models to estimate hazard ratios with 95% confidence intervals (CI) for survival analyses among those with a minimum of 6 months follow-up time with outcomes right-censored at 10 years. Data were analyzed between February 2022 and April 2022.
Results
Cohort characteristics
We identified 837 consecutive ILC tumors occurring in 813 patients (24 bilateral cases) between 1996 and 2019 (Fig. 1). Of these, we excluded cases with de novo metastatic disease (n = 14), those missing ER or PR status (n = 82), and those with ER positivity < 10% (n = 34), leaving 707 cases left for analysis in our study cohort (Table 1). Most tumors had classic ILC histology (n = 592), with some tumors having mixed ductal-lobular features (n = 52) or other histologic variants of ILC (n = 63). Pleomorphic histology was identified in 68 tumors. Of those pleomorphic cases, 17 were classic ILC with pleomorphic features, 1 was a mixed ILC/IDC, 1 was alveolar ILC, and the remaining 49 were categorized only as pleomorphic.
Overall, the mean age at diagnosis was 59.6 years (range 21–91), and most patients had a body mass index (BMI) in the range of 18.5–25 kg/m2 (51.62%). There were 436 (63.2%) patients with pathologic stage I disease, 168 (24.4%) with stage II, and 86 (12.5%) with stage III disease. Most tumors were grade 2 (n = 473, 68.1%), with 189 (27.2%) being grade 1 and 33 (4.8%) being grade 3. A small proportion of cases were HER2 positive [31 of 679 with data available (4.6%)], which was consistent with prior literature [11]. The mean follow-up time was 7.4 years [standard deviation (SD) 5.9].
Estrogen and progesterone receptor status
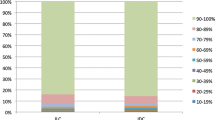
There were 522 (73.8%) cases with high ER (≥ 90% positive nuclei), 107 (15.1%) with medium ER staining (70–89% positive nuclei), and 78 (11.0%) with low ER staining (10–69% positive nuclei) (Table 2). Regarding progesterone receptor status, there were 478 (67.6%) with PR high (21–100% positive nuclei) and 229 (32.4%) with low PR (0–20% positive nuclei) (Table 2).
When combining ER and PR status, 366 cases (51.8%) were high for both, while 308 (43.6%) were low for either ER or PR, and 33 (4.7%) were low for both ER and PR (Table 1).
Associations of ER and PR status with clinicopathologic factors
Patients with high ER were significantly older than those with low or medium ER (mean age 60.6, 56.7, and 56.6 years for high, low, and intermediate respectively, p = 0.0006, Table 2). In contrast, those with high PR were significantly younger than those with PR negative or low (mean age 57.6 versus 63.6 years respectively, p < 0.0001). In combination, there was no statistical significance, however those high for both ER and PR (ER/PR high) were younger than those who were ER/PR low or ER/PR intermediate (58.8 years compared to 60.5 years in ER/PR low and 60.3 years in ER/PR intermediate, p = 0.23, Table 1). ER and PR status were not associated with BMI, either alone or in combination.
ER low status was associated with both PR low status and HER2 positivity. Of the ER low cases, 42.3% were also PR low, compared to 37.4% and 29.9% of the ER medium and ER high cases respectively, (p = 0.045). HER2 was overexpressed in 10.0%, 9.0%, and 2.9% of ER low, medium, and high cases respectively (p = 0.002) (Table 2). PR low status was also associated with HER2 overexpression, with 7.3% of PR low cases also being HER2 positive, compared to 3.3% of PR high cases (p = 0.017) (Table 2).
Tumor grade was associated with ER category, but not in the expected direction. ER low tumors were significantly more likely to be grade 1 than ER medium or high tumors (41.6%, 29.8% and 24.5% grade 1 among low, intermediate, and high, respectively, p = 0.03) (Table 2). In contrast, PR status was not associated with grade in this dataset. Interestingly, when ER and PR status were combined, those with both ER low and PR low status were least likely to be grade 2, and most likely to be either grade 1 or 3 (Table 1).
While ER status was not associated with pathologic stage, those with PR low status were more likely to have stage III disease than those with PR high status (17.3% versus 10.2% stage III respectively, p = 0.018) (Table 2). Neither ER nor PR category was associated with Ki67, the presence of lymphovascular invasion, or pleomorphic subtype. However, PR low cases were significantly less likely to have associated LCIS than PR high cases (62.6% versus 74.3%, p = 0.002) (Table 2).
Associations of ER and PR status with treatment
Overall, neoadjuvant chemotherapy was used in 85 (13.5%) cases in this study cohort, and adjuvant chemotherapy was used in 171 (24.6%) cases (Table 1). There were statistically significant differences in neoadjuvant therapy use for both ER and PR categories. For patients with ER low, medium, and high expression, neoadjuvant chemotherapy was used in 24.7%, 18.9%, and 10.5% respectively (p = 0.001) (Table 2). Among those with PR neg/low status, 36 (17.4%) received neoadjuvant chemotherapy compared to 11.6% of those with PR high status (p = 0.048) (Table 2).
Although all patients had hormone receptor positive disease, the use of adjuvant endocrine therapy was significantly lower in those with lower ER positivity. Among those with ER low tumors, only 65.8% received adjuvant endocrine therapy, compared to 77.1% of ER medium cases, and 81.4% of ER high (p = 0.007). There was no difference in the use of adjuvant endocrine therapy by PR low versus high category (Table 2).
The rates of mastectomy compared to breast-conserving surgery did not differ by ER or PR category; however, those with PR low status were significantly more likely to undergo lumpectomy without radiotherapy than those with PR high status (14.7% versus 22.4% respectively, p = 0.044). Interestingly, ER low status was associated with positive surgical margins at first excision (40.3% in ER low cases, 35.9% in ER medium cases, and 23.7% in ER high cases, p = 0.001). In a logistic regression model adjusting for tumor size and type of surgery, both ER low and ER medium status remained associated with significantly higher odds of positive margins compared to ER high status (odds ratio [OR] 2.4, 95% CI 1.4–3.9 and OR 1.9, 95% CI 1.2–3.0 for ER low and medium respectively). In contrast, PR status was not associated with positive margins at first excision.
Disease free survival
There were 88 patients who experienced a recurrence event during the study period. Of those who had recurrence events, 37 (42.0%) had local recurrence, 44 (50%) had distant recurrence, 5 (5.7%) had both local and distant recurrence, and 2 (2.3%) were missing the site of recurrence. ER level was not associated with DFS, using the log-rank test (Fig. 2a). However, low PR was associated with worse DFS than high PR (p = 0.024); this was also true when excluding non-classic and pleomorphic ILC cases (p = 0.0022) (Fig. 2b). Similarly, in combination, having either intermediate ER/PR (defined as either ER or PR low) or having low ER/PR (defined as both ER and PR low) was associated with significantly worse DFS, among all cases (p = 0.014, Fig. 2c), and also when excluding non-classic and pleomorphic ILC (p = 0.0199).
In a Cox proportional hazards model for DFS adjusting for age, stage, tumor grade, receipt of chemotherapy, receipt of adjuvant endocrine therapy, and HER2 status, ER category was not associated with DFS, but PR low status remained significantly associated with worse DFS (HR 2.2, p-value 0.003, 95% CI 1.3–3.8) (Table 3). This finding remained true when the multivariable model was restricted only to patients with classic ILC histology and no pleomorphic features. When evaluating the same multivariable model using time to local recurrence and time to distant recurrence as separate endpoints, PR low status was not associated with local recurrence, but was associated with distant recurrence (HR 2.7, p-value 0.007, 95% CI 1.3–5.4).
Of note, in a test of interaction between ER category and receipt of endocrine therapy, adjuvant endocrine therapy was associated with significantly improved DFS across ER low, medium, and high categories (Table 4).
Discussion
In this institutional cohort of 707 cases of ILC, we found that while most tumors had ER positivity ≥ 90%, more than a quarter had lower expression of ER, as defined by prior criteria.1 Nearly three-quarters of cases fell into the previously described category of ER high, 15.1% into ER medium, and 11% into ER low. Compared to a large population-based analysis of patients with ILC, our study had a lower proportion of patients with ER high positive tumors. A prior study of 2512 post-menopausal patients found that 84% of ILC cases were ER high, 11% were ER medium, and 4.6% were ER low [8]. The relative shift towards more cases with lower ER positivity in our cohort likely occurred because we included both pre-and post-menopausal patients. Consistent with this idea, we found a significant association between older age and ER high positivity.
There were interesting associations between ER status and clinicopathologic variables. Those with lower ER positivity were younger, were more likely to also have low PR, were more likely to be HER2 positive, but were also more likely to have low histologic grade. ER low and medium tumors were also more likely to have positive margins at surgical excision, but ultimately the type of local therapy and DFS did not differ by ER status.
In contrast, PR status was not only associated with clinicopathologic variables but was also associated with DFS on univariate and multivariate analyses. Those with PR low status were significantly older, were more likely to be HER2 positive, more likely to have a higher stage, less likely to have associated LCIS, and had a worse prognosis.
Analysis of cases in the West German Study Group Plan B trial did not demonstrate an association between PR negativity and DFS in those with ILC [12]. In contrast, we found a significant association between lower PR expression and worse DFS; since PR negativity is uncommon in ILC, combining negative and low PR cases allows for a larger sample size for comparison. Our findings are more consistent with large database studies that demonstrate a significant association between PR negativity and worse overall survival in patients with ILC [13].
Interestingly, we did not find an association between ER/PR expression and Ki67 in the 358 patients for whom Ki67 data were available. This is consistent with data from prior studies showing a lack of correlation between ER/PR status with Ki67 in patients with ILC, but a strong inverse relationship in those with invasive ductal carcinoma [10]. Wong et al.suggest that the disruption of β-catenin signaling in ILC prevents activation of the Wnt pathway, which usually results in increased Ki67, potentially making Ki67 a less reliable predictor of behavior in ILC [10]. Of note, Ki67 was not associated with DFS in this dataset.
While recent data have identified “ER-low positive” breast cancer as a unique subset that might require different treatment approaches, these analyses have not specifically evaluated lobular tumors. Given the low prevalence of ER staining < 10% in those with ILC, there are likely very few ILC tumors represented in these studies. This is consistent with our finding that of 837 ILC cases in our institutional database, only 10 (1.2%) had ER positivity 1–9%, making the classic definition of “ER-low” less relevant for ILC. However, as other investigators have demonstrated, there is a range of ER positivity within ILC tumors. Prior studies that predominantly included patients with invasive ductal carcinoma have shown associations between ER low status and worse DFS; our findings might differ because we used a different threshold for “ER low,” versus a differential impact of ER expression on ILC than IDC. While relatively lower ER positivity was associated with different clinicopathologic features in our study, it did not seem to drive outcomes, while PR low versus high status was an important predictor of DFS in this study [14].
It is important to note the overall limitations of our study. This is a single institution dataset which may not be generalizable. Additionally, retrospective analyses are subject to treatment bias and missing information. Adherence to endocrine therapy was not available in our dataset. Furthermore, there is no clear consensus on cut points for ER low, intermediate, and high categories with several studies reporting different definitions. In this analysis, we defined ER categories based on the publication by Truin et al. [8]. Molecular subtyping was not available in our study but might be informative to better understand the relationship between Luminal A and B status, particularly with respect to PR and Ki67, Recurrence Score, and to compare with the sensitivity to endocrine therapy (SETER/PR) index of endocrine-related transcriptional activity [15]. Interestingly, a large analysis found that PR status was prognostic in Luminal A but not Luminal B breast cancers [16]. Given the higher proportion of Luminal A cases among ILC, our findings may reflect this phenomenon [17]. This suggests that low PR status in ILC might identify a subset with poor prognosis despite low proliferative rates. In other work, molecular analysis of ILC cases have identified ILC specific subtypes that differ from the classic Luminal A/Luminal B framework, including the three categories of reactive-like, immune-related, and proliferative types and the two categories of immune-related and hormone-related ILC [18]− [19]. A better understanding of how the spectrum of ER/PR expression by IHC fits into these ILC specific molecular subtypes would be of interest.
Overall, our findings demonstrate the heterogeneity of hormone receptor expression in ILC. While ER low and medium tumors did not have different DFS than ER high tumors, they did differ in their association with clinicopathologic variables, suggesting the need for further study into drivers of tumor development and optimal treatment. In contrast, low PR expression was an independent predictor of worse DFS.
Conclusion
Our findings suggest there is wide clinicopathologic heterogeneity among hormone receptor status in ILC for both progesterone and estrogen. These findings broadly support the need for future research on the impact of relatively low ER and low PR in ILC to better understand these relationships and individualize treatment.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to protect patient confidentiality but are available redacted from the corresponding author on reasonable request.
References
Reinert T, Cascelli F, de Resende CAA, Gonçalves AC, Godo VSP, Barrios CH (2022) Clinical implication of low estrogen receptor (ER-low) expression in breast cancer. Front Endocrinol 13:1015388. https://doi.org/10.3389/fendo.2022.1015388
Allison KH et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 38(12):1346–1366. https://doi.org/10.1200/JCO.19.02309
Fei F, Siegal GP, Wei S (2021) Characterization of estrogen receptor-low-positive breast cancer. Breast Cancer Res Treat 188(1):225–235. https://doi.org/10.1007/s10549-021-06148-0
Luo C, Zhong X, Fan Y, Wu Y, Zheng H, Luo T (2022) Clinical characteristics and survival outcome of patients with estrogen receptor low positive breast cancer. Breast 63:24–28. https://doi.org/10.1016/j.breast.2022.03.002
Poon IK, Tsang JY, Li J, Chan S-K, Shea K-H, Tse GM (2020) The significance of highlighting the oestrogen receptor low category in breast cancer. Br J Cancer 123(8):1223–1227. https://doi.org/10.1038/s41416-020-1009-1
Xie Y, Yang L, Wu Y, Zheng H, Gou Q (2022) Adjuvant endocrine therapy in patients with estrogen receptor-low positive breast cancer: a prospective cohort study. Breast 66:89–96. https://doi.org/10.1016/j.breast.2022.09.008
Schrodi S et al (2021) Outcome of breast cancer patients with low hormone receptor positivity: analysis of a 15-year population-based cohort. Ann Oncol 32(11):1410–1424. https://doi.org/10.1016/j.annonc.2021.08.1988
Truin W, Roumen RMH, Siesling S, van de Vijver KK, Tjan-Heijnen VCG, Voogd AC (2017) Estrogen and progesterone receptor expression levels do not differ between lobular and ductal carcinoma in patients with hormone receptor-positive tumors. Breast Cancer Res Treat 164(1):133–138. https://doi.org/10.1007/s10549-017-4220-x
Prat A et al (2013) Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal a breast cancer. J Clin Oncol 31(2):203–209. https://doi.org/10.1200/JCO.2012.43.4134
Wong H et al (2014) Lobular breast cancers lack the inverse relationship between ER/PR status and cell growth rate characteristic of ductal cancers in two independent patient cohorts: implications for tumor biology and adjuvant therapy. BMC Cancer 14(1):826. https://doi.org/10.1186/1471-2407-14-826
Kee G-J et al (2020) Human epidermal growth factor receptor 2 positive rates in invasive lobular breast carcinoma: the Singapore experience. World J Clin Oncol 11(5):283–293. https://doi.org/10.5306/wjco.v11.i5.283
Christgen M et al (2020) Differential impact of prognostic parameters in hormone receptor–positive lobular breast cancer. Cancer 126(22):4847–4858. https://doi.org/10.1002/cncr.33104
Liu J, Chen K, Mao K, Su F, Liu Q, Jacobs LK (2016) The prognostic value of age for invasive lobular breast cancer depending on estrogen receptor and progesterone receptor-defined subtypes: a NCDB analysis. Oncotarget 7(5):6063–6073. https://doi.org/10.18632/oncotarget.5844
Kim MC, Park MH, Choi JE, Kang SH, Bae YK (2022) Characteristics and prognosis of estrogen receptor low-positive breast cancer. J Breast Cancer 25(4):318. https://doi.org/10.4048/jbc.2022.25.e31
Khan SA et al (2022) Early local therapy for the primary site in de novo stage IV breast cancer: results of a randomized clinical trial (E2108). J Clin Oncol 40(9):978–987. https://doi.org/10.1200/JCO.21.02006
Ono M, Tsuda H, Yoshida M, Shimizu C, Kinoshita T, Tamura K (2017) Prognostic significance of progesterone receptor expression in estrogen-receptor positive, HER2-negative, node-negative invasive breast cancer with a Low Ki-67 labeling index. Clin Breast Cancer 17(1):41–47. https://doi.org/10.1016/j.clbc.2016.06.012
Danzinger S et al (2021) Invasive lobular carcinoma: clinicopathological features and subtypes. J Int Med Res 49(6):030006052110170. https://doi.org/10.1177/03000605211017039
Ciriello G et al (2015) Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163(2):506–519. https://doi.org/10.1016/j.cell.2015.09.033
Michaut M et al (2016) Integration of genomic, transcriptomic and proteomic data identifies two biologically distinct subtypes of invasive lobular breast cancer. Sci Rep 6(1):18517. https://doi.org/10.1038/srep18517
Funding
Rita A Mukhtar was supported by the National Cancer Institute Award K08CA256047.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. Material preparation, data collection and analysis were performed by ENC and RAM. The first draft of the manuscript was written by ENC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Symmans’ disclosures include Stock and Other Ownership Interests: ISIS Pharmaceuticals, Delphi Diagnostics, Eiger BioPharmaceuticals. Consulting or Advisory Role: Merck, AstraZeneca. Research Funding: Pfizer (Inst). Patents, Royalties, Other Intellectual Property: Co-inventor, US Patent No. 11,459,617 “Targeted Measure of Transcriptional Activity Related to Hormone Receptors,” issued on October 4, 2022 (applicant proprietor: University of Texas MD Anderson Cancer Center). Uncompensated Relationships: Delphi Diagnostics. A. Jo Chien’s disclosures include research funding from Merck, Puma, Amgen, and Seattle Genetics. The remaining authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Our study was approved by University of California San Francisco Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Clelland, E.N., Rothschild, H.T., Patterson, A. et al. Quantifying hormone receptor status in lobular breast cancer in an institutional series: the relationship between estrogen and progesterone receptor status and outcomes. Breast Cancer Res Treat 202, 367–375 (2023). https://doi.org/10.1007/s10549-023-07059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07059-y