Abstract
Purpose
To analyze the association between the Neighborhood Deprivation Index (NDI) and clinical outcomes of locoregional breast cancer (BC).
Methods
Surveillance, Epidemiology and End Results (SEER) database is queried to evaluate overall survival (OS) and disease-specific survival (DSS) of early- stage BC patients diagnosed between 2010 and 2016. Cox multivariate regression was performed to measure the association between NDI (Quintiles corresponding to most deprivation (Q1), above average deprivation (Q2), average deprivation (Q3), below average deprivation (Q4), least deprivation (Q5)) and OS/DSS.
Results
Of the 88,572 locoregional BC patients, 27.4% (n = 24,307) were in the Q1 quintile, 26.5% (n = 23,447) were in the Q3 quintile, 17% (n = 15,035) were in the Q2 quintile, 13.5% (n = 11,945) were in the Q4 quintile, and 15.6% (n = 13,838) were in the Q5 quintile. There was a predominance of racial minorities in the Q1 and Q2 quintiles with Black women being 13–15% and Hispanic women being 15% compared to only 8% Black women and 6% Hispanic women in the Q5 quintile (p < 0.001). In multivariate analysis, in the overall cohort, those who live in Q2 and Q1 quintile have inferior OS and DSS compared to those who live in Q5 quintile (OS:- Q2: Hazard Ratio (HR) 1.28, Q1: HR 1.2; DSS:- Q2: HR 1.33, Q1: HR 1.25, all p < 0.001).
Conclusion
Locoregional BC patients from areas with worse NDI have poor OS and DSS. Investments to improve the socioeconomic status of areas with high deprivation may help to reduce healthcare disparities and improve breast cancer outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common malignancy in women globally and the most common cause of cancer-associated mortality [1]. Several demographics, clinicopathological factors including size, grade of the tumor, lymph node (LN) status, hormone receptor status, metastasis, age, and comorbidities of the patients are known to be associated with BC survival [2,3,4]. In addition to this, racial and ethnic backgrounds are also associated with breast cancer survival; Black women have 40% higher age-adjusted BC mortality than non-Hispanic White women [3, 5]. The socioeconomic status (SES) of an individual and the neighborhood consists of multiple variables including income, education, occupation, living conditions which have been reported in relation to the survival of various cancers [6,7,8]. Neighborhood deprivation index (NDI) is a validated statistical measurement tool to assess the level of disadvantage within the specific neighborhood. It provides a quantitative measure of socio-economic status of community based on various indicators such as education, employment, income, living condition and basic access to services [9].
Reducing racial and ethnic and socioeconomic disparities in the access to health care have long been a major health policy goal in the United States (US). SES of the individual and neighborhood plays an important role in patient’s access to the health system [10]. Owing to the inequalities in opportunities, education, income, and developmental infrastructures, the areas with underprivileged individual and neighborhood SES may be associated with poor prognosis of certain malignancies and worse outcomes through multiple pathways [10, 11]. Patients with low individual SES may not be able to adhere to cancer screening guidelines, which may occur due to their lack of awareness of diseases or access to screening/prevention methods, lack of insurance or other cost-barriers, and/or mistrust of physicians/health sector [12, 13]. In addition to this, socioeconomically underprivileged neighborhoods lack comprehensive healthcare resources, established referral systems, adequate social support, resources to promote healthy lifestyle, and adequate transportation system to access to healthcare from the diagnosis to survivorship [11, 14, 15]. The myriad events rooting from low SES affect cancer-related mortality and morbidity in vulnerable populations.
Although there were some studies done in the past evaluating the association of individual SES with the survival of cancer, epidemiological studies aiming at the association of SES and geographical variation in BC outcomes are limited. Given the fact that most of the factors owing to low socioeconomic conditions are modifiable, it is very relevant to understand them and develop strategies to mitigate health disparities which aid us in improving health-related outcomes. In our study, we examine the association of neighborhood deprivation with the BC-related outcomes in patients with locoregional BC in the US.
Methodology
Data sources
Neighborhood deprivation index
In our analysis, we used the NDI which encompasses various factors such as wealth and income, education, occupation, and housing conditions. The NDI for each census tract in the US was created using factor analysis, which identified key variables from 13 measures from the above dimensions proposed by Roux and Mair in their study assessing the contribution of neighborhood or residential environments to social and ethnic inequalities in health [16].
“The key variables that are used from wealth and income are median household income, percent of household receiving dividends interest or rental income, percent of households receiving public assistance, median home value, percent of families with incomes below the poverty level. The variables from other dimensions are as follows: education (percent with a high school degree or higher; percent with a college degree or higher), occupation (percent in a management, business, science, or arts occupation; percent unemployed), and housing conditions (percent of households that are female-headed with any children under 18; percent of housing units that are owner occupied; percent of households without a telephone; percent of households without complete plumbing facilities) [9]. NDI values range from -3.6 to + 2.8 and higher values indicate more neighborhood deprivation which implies lower socioeconomic status”. We used the NDI quintiles weighted by the tract population for the analysis. The first NDI quintile corresponds to most deprivation (Q1), second quintile (above average deprivation- Q2), third quintile (average deprivation- Q3), fourth quintile (below average deprivation (Q4)) and fifth quintile corresponds to least deprivation (Q5) [17].
Patient selection
We queried the Surveillance, Epidemiology and End Results (SEER) registry November 2021 submission database which covers approximately 48% of the US population for our study. We included locoregional BC pts (clinical stage group I, II, III), aged > = 18 years, who were diagnosed from 2010 to 2016, and studied the overall survival (OS) and disease-specific survival (DSS) of BC in association with NDI. Patients were selected from 2010 to 2016 which allowed inclusion of patients with accurate HER2-neu status as accurately captured in SEER from 2010 onwards and adequate 5 years follow up. We excluded patients with unknown or missing data for each variable studied, or clinical/pathological evidence of distant metastases at the time of initial diagnosis. The flow diagram depicting patient selection is shown in Fig. 1. Institutional review board review was exempted as the data were deidentified and from publicly available databases upon request.
Statistical analysis
The demographical and clinical characteristics of patients by NDI were tabulated by summary statistics. The mean, median, standard deviation, and range were used for continuous variables and the Kruskal-Wallis test was used for comparisons. For the categorical variables, frequencies and relative frequencies were compared using the chi-square test. The median, 3-year, 5-year OS and DSS were summarized by NDI using standard Kaplan-Meier methods.
Cox multivariate regression modeling was performed to test the association between NDI and OS, DSS, with adjustment for age, race, stage, grade, insurance status, surgery, radiation, and chemotherapy (CT). Subset analysis was done based on the BC subtypes (Estrogen receptor and/or progesterone receptor positive HER2-neu negative (HR+), HER2-neu-positive (HER2+), and triple-negative breast cancer (TNBC). All statistics were performed using SAS software version 9.4 (SAS Institute Inc.) and significance testing was 2-sided at p < 0.05. Data were analyzed from June 1, 2022 through July 15, 2022.
Results
Patient demographics
The baseline characteristics of the overall cohort are shown in Table 1. Of the 88,572 locoregional BC patients, 27.4% (n = 24,307) were in the most deprivation (Q1) quintile, 17% (n = 15,035) were in the above average deprivation (Q2) quintile, 26.5% (n = 23,447) were in the average deprivation (Q3) quintile, 13.5% (n = 11,945) were in the below average deprivation (Q4) quintile, and 15.6% (n = 13,838) were in the least deprivation (Q5) quintile. The median age of patients in the Q5 quintile was 59 years and Q1 quintile was 61 years (p < 0.001). There was a predominance of racial (p < 0.001) and ethnic (p < 0.001) minorities in the most deprived (Q1) quintile (12.9% Black, 14.8% Hispanic) compared to least deprived (Q5) quintile (8.2% Black, 6.0% Hispanic) (Table 1). There was a higher percentage of uninsured patients in the Q1 quintile compared to the Q5 quintile (2.2% vs. 1.7%, p < 0.001). There were more rural areas in Q1 quintile compared to Q5 quintile (25.9% vs. only 0.7%, p < 0.001). Patients with stage III and grade III disease were observed to be higher in Q1 quintile compared to Q5 quintile (Stage III: 28.7% vs. 14.2%, Grade III: 34% vs. 31.9%, p < 0.001) and a greater percentage of patients received CT in Q1 quintile compared to Q5 quintile (44.6% vs. 42.1%, p < 0.001). However, fewer patients underwent surgery and radiation in the Q1 compared to the Q5 quintile, with 96.1% and 49.7% of patients undergoing surgery and radiation in Q1 quintile compared to 97.1% and 56.5% in the Q5 quintile (p < 0.001 for both, Table 1). There was a higher percentage of aggressive cancers such as TNBC and HER2 + BC in Q1 quintile compared to Q5 quintile (14.5%, 17.7% vs. 11.7%, 16.5% respectively, p < 0.001). The baseline characteristics were stratified by the subtype of breast cancer as shown in Tables 2, 3 and 4. It was observed that the patterns are similar in all the subtypes as observed in the overall cohort except that the patients who received chemotherapy for the locoregional BC were higher in the Q5 when compared to the Q1 in both TNBC and HER2 + BCs.
Kaplan-Meier survival estimates
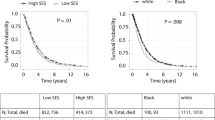
On univariate analysis, after a median follow-up of 44 months, the 5-year OS rate of the overall cohort was 87%. The 5-year OS of the locoregional BC patients who live in the Q1 and Q2 quintile was lower when compared to those who live in the Q5 quintile (85%, 84% vs. 89%, p < 0.001). The DSS of the overall cohort also followed a similar pattern (DSS of Q1, Q2 vsQ5: 92%, 91% vs. 94%, p < 0.001) (Table 5; Fig. 2).
In subset analysis stratified by the BC subtypes, the 5-year OS and DSS were lower in the Q1 and Q2 quintiles compared to the Q5 quintile in all the subtypes of BC (HR+, HER2 + and TNBC). However, the 5-year DSS rate was not significantly different in the HR + subtype (Q1, Q2 vs. Q5: 95%, 95% vs. 96%, p < 0.001). (Table 5; Fig. 3).
Kaplan-Meier Curves of overall and disease-specific survival by NDI for locoregional breast cancer subtypes
NDI: Neighborhood deprivation index, Avg: Average, N: Number, HR+ : Hormone receptor-positive human epidermal growth factor 2- negative, HER2 + : Human epidermal growth factor receptor 2 positive
Multivariate survival analysis
In multivariate analysis after adjusting for socio-demographic, clinical, and treatment variables, in the overall cohort, those who live in Q2 quintile and Q1 quintile have inferior OS and DSS when compared to those who live in Q5 quintile (OS in Q2: Hazard Ratio (HR) 1.28; OS in Q1: HR 1.2; DSS in Q2: HR 1.33; DSS in Q1: HR 1.25, all p < 0.001). In the subset analysis, similar results for OS and DSS by NDI were observed in hormone receptor positive HER2 negative (HR+) and HER2 + subtypes, but not in TNBC (Fig. 4). In a separate multivariate cox regression model, age, race, insurance status, sub-type, disease grade, surgery, radiation, and chemotherapy were found to be independently associated with OS and DSS (Tables 6 and 7 respectively). The OS and DSS in locoregional BC by race are given in Table 8.
Multivariate survival analysis in the locoregional overall cohort: overall survival and disease-specific survival
HR (95% CI): Hazard ratio (95% confidence interval), Avg: Average, TNBC: Triple negative breast cancer, HR+: Hormone receptor-positive human epidermal growth factor 2- negative, HER2+ : Human epidermal growth factor receptor 2 positive
Discussion
Our study focuses on locoregional BC as its treatment requires access to health care systems that are less available in underprivileged neighborhoods. In our study, we found that the deprivation index of the neighborhoods was in significant association with BC survival. Our analysis showed that the OS and DSS of patients with locoregional BC were lower for those who live in socioeconomically underprivileged neighborhoods compared to those who live in affluent neighborhoods. The survival differences were observed among all subtypes of BC. The differences in survival persisted even after adjusting for demographic, clinical, and treatment factors that could affect BC survival. On multivariate analysis, the mortality difference among the patients living in different socioeconomic areas was statistically significant within the overall cohort, HR + and HER2 + BC subtypes, but not within the TNBC subtype. This could be explained by the aggressive nature of TNBC. As TNBC is very aggressive and has high relapse rate [18], the survival of patients with TNBC could be poor regardless of their socioeconomic status.
Understanding the impact of neighborhood deprivation on BC survival will facilitate the development and implementation of policies and prioritize investments in communities with high deprivation scores. This could improve the socioeconomic conditions which could eventually improve clinical outcomes [19]. Several factors in the neighborhood influence the health of an individual directly, as well as indirectly: poverty, access to the health care system, transportation system, housing quality, unemployment, environmental pollution including air and water pollution, neighborhood hygiene, waste management system, crime rates, racial composition, educational system, tobacco availability and marketing, access to healthy food [20,21,22,23]. These along with the factors that affect the individual such as marital status, family/social support, co-morbidities, mental health, nutritional status, healthy lifestyle, insurance status, and educational status play an inevitable role in the survival outcomes of malignancies. Studies have shown that prolonged and cumulative exposure to the above-mentioned deprivation-associated stressors can induce chronic inflammation which is one of the etiologies behind cancer development [24, 25]. Therefore, a proper understanding of the deprivation factors of an individual and their neighborhood is essential to plan interventions to reduce the burden of cancer mortality.
Our study adds to the existing literature in multiple ways. This study is the first to our knowledge to use a national database to examine the association between neighborhood deprivation with the clinical outcomes of locoregional BC; most prior studies used regional databases. Prior studies showed racial disparities in BC-related outcomes in the US and minoritized groups tend to have higher BC-related mortality [22]. In a study by Luningham et al. which examined the association between racial disparities and SES in BC survival between Black and White women across Georgia, it was found that Black women with BC had higher mortality than White women, but this disparity was not explained by the socioeconomic deprivation of their residential area. White patients living in socioeconomically affluent areas had lower rates of BC mortality compared to those who reside in underprivileged neighborhoods [26]. We observed similar results in our study: We observed similar results in our study. White BC patients living in socioeconomically underprivileged areas had higher mortality compared to Whites living in socioeconomically affluent areas. However, this difference in the mortality was not observed among Black BC patients. This is an interesting finding that may need validation in future studies. This difference leads us to speculate that irrespective of area of residence, Black breast cancer patients continue to have worse outcomes. This may be either due to cultural factors precluding Black patients to seek healthcare, mistrust for the system, lack of insurance, or could be due to inherent aggressive disease that even with access to the above facilities, the natural biology of the disease continues to be aggressive [27]. This clearly highlights the need for both better treatments for Black patients as well as more attention at a policy level for Black patients.
Our study finding critically shows the important role of area of residence on clinical outcomes, and thus emphasizing that socioeconomic factors of the neighborhood play a vital role in determining clinical outcomes. There were several studies conducted to understand the reason behind the observed racial disparities. One of them was attributed to poor neighborhoods. Black and Hispanic women are more likely to live in poor neighborhoods and Black patients were found to live in neighborhoods with high poverty rates and this difference persists even after adjusting for their income status [28]. In our study, we found that Black and Hispanic women with BC were more commonly residing in the underprivileged neighborhoods compared to socioeconomically affluent neighborhoods; however, the disparities in BC-related mortality of the patients from these socioeconomically different neighborhoods was not observed among Black patients and persisted even after accounting for the racial disparities.
Similarly, patients without insurance were found more commonly in the underprivileged neighborhoods and those neighborhoods had more rural areas. Advanced BCs (higher stage and grade) and aggressive BC such as HER2 + and TNBC were predominantly found in the underprivileged neighborhoods compared to the affluent neighborhoods. In our study, BC patients from affluent neighborhoods received more surgical and radiation treatments which can be explained by the higher percentages of urban areas in these regions with better facilities for treatments, and better referral systems. In a study by Fwelo et al., it was found that Black and Hispanic women were more likely to undergo mastectomy compared to Whites [29]. This discrepancy could be attributed to the fact that breast cancer is often diagnosed when locally advanced in Black women, indicating limited access to early detection through adequate screening and precluding lumpectomy [27, 29]. Additionally, cultural preferences, low healthcare literacy, and transportation barriers may contribute to limited healthcare access, potentially influencing the decision to avoid radiation treatment [27, 30]. Our study showed that in the overall BC cohort, patients who received chemotherapy were slightly higher in the underprivileged neighborhoods than in the affluent neighborhood. One possible explanation for this is that the advanced diseases that requires chemotherapy was more prevalent in the underprivileged neighborhood regions. This could also be attributed to several other factors, such as low healthcare education, leading to misconceptions about medical information, as well as limited access to tests (due to insurance issues) that predict the benefits of adjuvant chemotherapy, like the Oncotype DX 21-gene expression assay, which can potentially result in overtreatment among racial minorities [27, 31]. Nevertheless, the disparities in BC-related mortality remain unaffected when adjusted for the demographic, clinical, pathological, and treatment-related factors such as age, race, stage, grade, insurance, surgery, radiation, and chemotherapy. This suggests that additional factors related to neighborhood SES that are not captured by the NDI play an important role in BC-related outcomes. The access to genetic and somatic testing which are important for deciding the appropriate treatments in BC might be limited to patients from poor neighborhood which could have impacted their survival.
Poverty, unhealthy food habits, decreased access to healthy food, environmental pollution, increased advertising of tobacco in poor neighborhood leads to increased incidence of various cancers in patients from these neighborhoods [20, 21, 23, 32, 33]. Furthermore, the transportation barriers, decreased access to better comprehensive cancer centers with standard of care treatments or novel clinical trials, poor nutritional and educational status of patients, financial toxicity associated with cancer treatment leads to increased cancer-related mortality in socioeconomically poor neighborhoods [11, 15, 34, 35]. In addition to this, poor environmental hygiene and pollution can add to the increased mortality by causing infections in cancer patients who are already immunocompromised due to cancer and associated treatments [36]. In a patient-reported outcomes study in advanced cancers, it was found that patients from socioeconomically underprivileged areas have a higher level of anxiety [37]. Factors such as anxiety, depression, and poor social support which are subjective measures of poor mental health are not accounted for in any of the tools to measure the neighborhood/individual SES and have been shown to be associated with cancer-related mortality [14, 37, 38]. Disparities in BC survival related to neighborhood SES reflect the systematic barriers in policies related to health care, education, employment, insurance, environment, and judicial system. Our study findings may support restructuring the policies, to implement new policies and investments in socioeconomically underprivileged neighborhoods which would help to decrease inequalities in opportunities, improve healthcare facilities, and increase access to timely cancer treatments.
Our study has many strengths and certain limitations. We used large real-world data to assess the impact of neighborhood deprivation on clinical outcomes of BC patients. These data capture more than 50% of the US population, and therefore, the results are generalizable. We adjusted for multiple factors that are known to influence survival, including racial distribution, demographic factors, clinicopathological factors of the disease [39]. Although NDI is a comprehensive tool to assess socioeconomic disadvantage, it may not capture all the factors associated with neighborhood SES. We could not assess the influence of several neighborhood factors that may contribute to the mortality of BC such as access to transportation, environmental: air, water pollution, poverty level, accessibility to healthy food, and crime rate of the neighborhood. As we do not have one comprehensive tool to assess the socioeconomic status of neighborhoods and individuals together, future studies warranting the combination of multiple indices such as area deprivation index, Yost index might be beneficial. As it is a retrospective national database study, several individual factors that can affect the mortality rates of BC such as comorbidities of patients, social support, details of factors such as anxiety, and depression that can affect the mental and physical condition of the patients were not collected. Incompleteness of individual-level data collected on cancer risk and treatment, and incomplete values for several variables collected from multiple registries were other limitations. Further tumor recurrence data, specific details on the type, dose, and duration of chemotherapy, radiation, oral chemotherapy, targeted agents were not available in the SEER database and these factors could have been associated with the mortality of BC.
Moving forward, it is crucial for professional societies and cancer institutes to take the initiative to design strategies that address breast cancer disparities stemming from the socioeconomic status of neighborhoods. Some potential initiatives include identifying underprivileged areas in communities and providing additional facilities to these patients, such as offering free transportation and, organizing mobile units equipped with imaging technologies to visit underprivileged areas regularly. Additionally, addressing the climate gap through federal support to rebuild infrastructure, reduce sources of pollution, and ensure accessible and affordable clean energy sources can be another strategy to alleviate economic injustice [40]. Restructuring the health system to address the racial wealth gap, promoting equity and inclusion by ensuring adequate access to clinical trials for everyone, supporting research focused on preventing and treating aggressive breast cancer subtypes (more prevalent in racial minorities, such as Blacks), and ensuring universal access to healthcare, are key strategies that can be adopted to decrease healthcare disparities and reduce mortality rates from breast cancer [5, 41, 42].
Conclusion
The findings from our study suggest that neighborhood deprivation is significantly and independently associated with worse clinical outcomes among patients with BC in the US. Locoregional BC patients from areas with worse NDI have poor OS and DSS, after accounting for demographic, clinicopathological, and treatment-related factors. Identification of these poor-resource neighborhoods is critical to guide investments in these neighborhoods and implement policies focusing on improving the SES of these areas with high deprivation to reduce healthcare disparities and improve breast cancer outcomes. Future studies are warranted to understand the factors affecting the neighborhood socioeconomic status other than what is mentioned in our study and to assess their relationship with BC-related survival. The data from these studies might be extrapolated to other cancers which would help us to improve the quality of life of patients and cancer-related mortalities.
Data Availability
All data utilized in this article is available in public datasets such as SEER and NCI Neighborhood deprivation index. Data analyzed during this study are included in this published article and its Appendix.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Dong G, Wang D, Liang X, Gao H, Wang L, Yu X, Liu J (2014) Factors related to survival rates for breast cancer patients. Int J Clin Exp Med 7:3719–3724
Roy AM, Jiang C, Yao S, Perimbeti S, Gandhi S (2022) Does race influence long-term outcomes after neoadjuvant chemotherapy in breast cancer: a National Cancer database analysis. J Clin Oncol 40:606–606. https://doi.org/10.1200/JCO.2022.40.16_suppl.606
Seedhom AE, Kamal NN (2011) Factors affecting survival of women diagnosed with breast cancer in El-Minia Governorate, Egypt. Int J Prev Med 2:131–138
Jatoi I, Sung H, Jemal A (2022) The emergence of the racial disparity in U.S. breast-Cancer mortality. N Engl J Med 386:2349–2352. https://doi.org/10.1056/NEJMp2200244
Shavers VL (2007) Measurement of socioeconomic status in health disparities research. J Natl Med Assoc 99:1013–1023
Cella DF, Orav EJ, Kornblith AB, Holland JC, Silberfarb PM, Lee KW, Comis RL, Perry M, Cooper R, Maurer LH et al (1991) Socioeconomic status and cancer survival. J Clin Oncol 9:1500–1509. https://doi.org/10.1200/JCO.1991.9.8.1500
Singh GK, Jemal A (2017) Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health 2017, 2819372, doi:https://doi.org/10.1155/2017/2819372
Neighborhood Deprivation Index (2023) https://www.gis.cancer.gov/research/adopt.html. Accessed on February 15,
Lazar M, Davenport L (2018) Barriers to Health Care Access for low income families: a review of literature. J Community Health Nurs 35:28–37. https://doi.org/10.1080/07370016.2018.1404832
Bhatt J, Bathija P (2018) Ensuring Access to Quality Health Care in Vulnerable Communities. Acad Med 93:1271–1275. https://doi.org/10.1097/ACM.0000000000002254
Ahern MM, Hendryx MS (2003) Social capital and trust in providers. Soc Sci Med 57:1195–1203. https://doi.org/10.1016/s0277-9536(02)00494-x
Shah SA, Mahmood MI, Ahmad N (2020) Low socioeconomic Status Associated with Poor Cancer screening perceptions in Malaysia: analysis of determinant of Health among General Population. Asian Pac J Cancer Prev 21:3137–3144. https://doi.org/10.31557/APJCP.2020.21.11.3137
Moskowitz D, Vittinghoff E, Schmidt L (2013) Reconsidering the effects of poverty and social support on health: a 5-year longitudinal test of the stress-buffering hypothesis. J Urban Health 90:175–184. https://doi.org/10.1007/s11524-012-9757-8
Kirkwood MK, Bruinooge SS, Goldstein MA, Bajorin DF, Kosty MP (2014) Enhancing the american society of clinical oncology workforce information system with geographic distribution of oncologists and comparison of data sources for the number of practicing oncologists. J Oncol Pract 10:32–38. https://doi.org/10.1200/JOP.2013.001311
Diez Roux AV, Mair C (2010) Neighborhoods and health. Ann N Y Acad Sci 1186:125–145. https://doi.org/10.1111/j.1749-6632.2009.05333.x
Neighborhood Deprivation Index Codebook (2023) https://www.gis.cancer.gov/research/NeighDeprvIndex_Codebook.pdf. Accessed on February 15,
Gupta RK, Roy AM, Gupta A, Takabe K, Dhakal A, Opyrchal M, Kalinski P, Gandhi S (2022) Systemic therapy de-escalation in Locoregional Triple-Negative breast Cancer: Dawn of a new era? Cancers (Basel) 14. https://doi.org/10.3390/cancers14081856
Lyons MJ, Fernandez Poole S, Brownson RC, Lyn R (2022) Place is power: investing in Communities as a systemic leverage point to reduce breast Cancer disparities by race. Int J Environ Res Public Health 19. https://doi.org/10.3390/ijerph19020632
Arcaya MC, Tucker-Seeley RD, Kim R, Schnake-Mahl A, So M, Subramanian SV (2016) Research on neighborhood effects on health in the United States: a systematic review of study characteristics. Soc Sci Med 168:16–29. https://doi.org/10.1016/j.socscimed.2016.08.047
Diez Roux AV (2016) Neighborhoods and health: what do we know? What should we do? Am J Public Health 106:430–431. https://doi.org/10.2105/AJPH.2016.303064
Hunt BR, Hurlbert MS (2016) Black:white disparities in breast cancer mortality in the 50 largest cities in the United States, 2005–2014. Cancer Epidemiol 45:169–173. https://doi.org/10.1016/j.canep.2016.07.018
Lee JG, Henriksen L, Rose SW, Moreland-Russell S, Ribisl KM (2015) A systematic review of Neighborhood Disparities in Point-of-sale Tobacco Marketing. Am J Public Health 105:e8–18. https://doi.org/10.2105/AJPH.2015.302777
Duru OK, Harawa NT, Kermah D, Norris KC (2012) Allostatic load burden and racial disparities in mortality. J Natl Med Assoc 104:89–95. https://doi.org/10.1016/s0027-9684(15)30120-6
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867. https://doi.org/10.1038/nature01322
Luningham JM, Seth G, Saini G, Bhattarai S, Awan S, Collin LJ, Swahn MH, Dai D, Gogineni K, Subhedar P et al (2022) Association of race and area deprivation with breast Cancer Survival among Black and White Women in the state of Georgia. JAMA Netw Open 5:e2238183. https://doi.org/10.1001/jamanetworkopen.2022.38183
Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, Alo RA, Payton M, Tchounwou PB (2019) Health and racial disparity in breast Cancer. Adv Exp Med Biol 1152:31–49. https://doi.org/10.1007/978-3-030-20301-6_3
Logan JR (2011) Separate and unequal: the Neighborhood gap for blacks, Hispanics and Asians in Metropolitan America. Project US2010 report
Fwelo P, Nwosu KOS, Adekunle TE, Afolayan O, Ahaiwe O, Ojaruega AA, Nagesh VK, Bangolo A (2023) Racial/ethnic and socioeconomic differences in breast cancer surgery performed and delayed treatment: mediating impact on mortality. Breast Cancer Res Treat 199:511–531. https://doi.org/10.1007/s10549-023-06941-z
Johnson KS, Elbert-Avila KI, Tulsky JA (2005) The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc 53:711–719. https://doi.org/10.1111/j.1532-5415.2005.53224.x
Roberts MC, Kurian AW, Petkov VI (2019) Uptake of the 21-Gene assay among women with Node-Positive, hormone receptor-positive breast Cancer. J Natl Compr Canc Netw 17:662–668. https://doi.org/10.6004/jnccn.2018.7266
Hall JM, Szurek SM, Cho H, Guo Y, Gutter MS, Khalil GE, Licht JD, Shenkman EA (2022) Cancer disparities related to poverty and rurality for 22 top cancers in Florida. Prev Med Rep 29:101922. https://doi.org/10.1016/j.pmedr.2022.101922
Zhang FF, Cudhea F, Shan Z, Michaud DS, Imamura F, Eom H, Ruan M, Rehm CD, Liu J, Du M et al (2019) Preventable Cancer Burden Associated with Poor Diet in the United States. JNCI Cancer Spectr 3:pkz034. https://doi.org/10.1093/jncics/pkz034
Shariff-Marco S, Yang J, John EM, Sangaramoorthy M, Hertz A, Koo J, Nelson DO, Schupp CW, Shema SJ, Cockburn M et al (2014) Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and breast Cancer study. Cancer Epidemiol Biomarkers Prev 23:793–811. https://doi.org/10.1158/1055-9965.EPI-13-0924
Zafar SY (2016) Financial Toxicity of Cancer Care: It’s Time to Intervene. J Natl Cancer Inst 108. https://doi.org/10.1093/jnci/djv370
Kagan LJ, Aiello AE, Larson E (2002) The role of the home environment in the transmission of infectious diseases. J Community Health 27:247–267. https://doi.org/10.1023/a:1016378226861
Rosenzweig MQ, Althouse AD, Sabik L, Arnold R, Chu E, Smith TJ, Smith K, White D, Schenker Y (2021) The Association between Area Deprivation Index and patient-reported outcomes in patients with Advanced Cancer. Health Equity 5:8–16. https://doi.org/10.1089/heq.2020.0037
Roy AM, Konda M, Warrier AM, Arnaoutakis K (2019) Depression in lung cancer patients: a nationwide analysis. J Clin Oncol 37:83–83. https://doi.org/10.1200/JCO.2019.37.31_suppl.83
Keegan TH, Kurian AW, Gali K, Tao L, Lichtensztajn DY, Hershman DL, Habel LA, Caan BJ, Gomez SL (2015) Racial/ethnic and socioeconomic differences in short-term breast cancer survival among women in an integrated health system. Am J Public Health 105:938–946. https://doi.org/10.2105/AJPH.2014.302406
Morello-Frosch R, Obasogie OK (2023) The Climate gap and the Color line - racial Health Inequities and Climate Change. N Engl J Med 388:943–949. https://doi.org/10.1056/NEJMsb2213250
South E, Venkataramani A, Dalembert G (2022) Building Black Wealth - the role of Health Systems in closing the gap. N Engl J Med 387:844–849. https://doi.org/10.1056/NEJMms2209521
Boulware LE, Corbie G, Aguilar-Gaxiola S, Wilkins CH, Ruiz R, Vitale A, Egede LE (2022) Combating Structural Inequities - Diversity, Equity, and inclusion in clinical and translational research. N Engl J Med 386:201–203. https://doi.org/10.1056/NEJMp2112233
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers KL2TR001413 and UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
Arya Mariam Roy and Shipra Gandhi contributed to the study conception and design. Data acquisition, interpretation of data and statistical analysis was performed by Arya Mariam Roy, Anthony George, Kristopher Attwood and Shipra Gandhi. Initial draft of the manuscript was written by Arya Mariam Roy. Initial draft was reviewed and edited by Archit Patel and Sabah Alaklabi. All authors commented on revision of manuscript for important intellectual content. The project was conducted under the supervision of Shipra Gandhi. All authors have read and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study does not require Institutional Review Board (IRB) approval as all data used for analysis are deidentified and from publicly available databases.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roy, A.M., George, A., Attwood, K. et al. Effect of neighborhood deprivation index on breast cancer survival in the United States. Breast Cancer Res Treat 202, 139–153 (2023). https://doi.org/10.1007/s10549-023-07053-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07053-4