Abstract
Purpose
Prescribing NAC for breast cancer is a pragmatic treatment strategy for several reasons; however, certain patients suffer chemotherapy-induced toxicities. Unfortunately, identifying patients at risk of toxicity often proves challenging. MiRNAs are small non-coding RNA molecules which modulate genetic expression. The aim of this study was to determine whether circulating miRNAs are sensitive biomarkers that can identify the patients likely to suffer treatment-related toxicities to neoadjuvant chemotherapy (NAC) for primary breast cancer.
Methods
This secondary exploratory from the prospective, multicentre translational research trial (CTRIAL ICORG10/11–NCT01722851) recruited 101 patients treated with NAC for breast cancer, from eight treatment sites across Ireland. A predetermined five miRNAs panel was quantified using RQ-PCR from patient bloods at diagnosis. MiRNA expression was correlated with chemotherapy-induced toxicities. Regression analyses was performed using SPSS v26.0.
Results
One hundred and one patients with median age of 55 years were recruited (range: 25–76). The mean tumour size was 36 mm and 60.4% had nodal involvement (n = 61) Overall, 33.7% of patients developed peripheral neuropathies (n = 34), 28.7% developed neutropenia (n = 29), and 5.9% developed anaemia (n = 6). Reduced miR-195 predicted patients likely to develop neutropenia (P = 0.048), while increased miR-10b predicted those likely to develop anaemia (P = 0.049). Increased miR-145 predicted those experiencing nausea and vomiting (P = 0.019), while decreased miR-21 predicted the development of mucositis (P = 0.008).
Conclusion
This is the first study which illustrates the value of measuring circulatory miRNA to predict patient-specific toxicities to NAC. These results support the ideology that circulatory miRNAs are biomarkers with utility in predicting chemotherapy toxicity as well as treatment response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Contemporary breast cancer management pragmatically uses neoadjuvant chemotherapy (NAC) as the standard of care in patients diagnosed with certain breast cancer molecular subtypes [1, 2]. While survival outcomes for patients with breast cancer are equivalent for those treated with adjuvant and NAC [3, 4], there are several advantages of prescribing NAC, including potential tumour downstaging, increasing patient eligibility for breast conservation surgery (BCS), and obtaining in vivo sensitivity of the tumour to treatment [5], which correlates with long-term survival [6]. Unfortunately, certain host and tumour factors make predicting treatment response challenging, and the risk of overtreatment is incessant [7]. Chemotherapeutic drugs are cytotoxic to human cells [8], and their administration adopts a untargeted approach, where therapies do not differentiate cancer cells from healthy host cells, leading to widespread toxicity [9]. Therefore, a proportion of patients receiving chemotherapies develop undesired treatment-related toxicities [10] and identifying such patients remains an ever-present challenge to the multidisciplinary team. Thus, the discovery of methods of identifying these patients is imperative to improve the care of our prospective patients being treated for cancer.
Micro ribonucleic acids (or miRNAs) are a contemporary class of small, non-coding ribonucleic acid (RNA) molecules which are estimated to be approximately 19–25 nucleotides in length [11]. It is now recognised that these biomolecules possess key modulatory roles in genetic expression by influencing the post-transcriptional degradation of messenger RNA [12, 13]. MiRNA expression profiles have been implicated as regulators of several cellular processes [14] and maintain their stability in several biological tissues, including tumour tissue, healthy host tissue, and human circulation [15]. Notwithstanding their ability to predict treatment response and estimate patient prognosis [16,17,18], there are emerging data suggesting miRNA may have roles in toxicities to cancer therapeutics, including chemoradiotherapies and immunomodulatory agents [19, 20]. Furthermore, the measurement of miRNAs may be performed relatively simply and inexpensively using real-time quantitative reverse transcriptase polymerase chain reaction (RQ-PCR) [21], which improves the clinical candidacy of these biomolecules as potential cancer and therapeutic biomarkers.
At present, there is a paucity of translational research studies which have successfully identified patients at an increased risk of chemotherapy-induced toxicities. The Cancer Trials Ireland—Irish Clinical Oncology Research Group 10/11 (CTRIAL ICORG 10/11) study was a prospective, multicentre clinical trial which recruited 120 patients indicated to undergo standard-of-care NAC from 8 treatment sites across the Republic of Ireland. In the primary analysis of the ICORG 10/11 trial, a predetermined miRNA panel was relatively quantified from bloods samples using RQ-PCR at several timepoints during NAC and correlated with treatment response to NAC [16, 22]. The current study is a secondary exploratory analysis of the ICORG 10/11 trial and, to the authors knowledge, is the first study to explore the clinical utility of miRNAs as circulatory biomarkers which may be useful in identifying patients who are at an increased risk of developing treatment-induced toxicities to NAC for primary breast cancer.
Methods
Study design
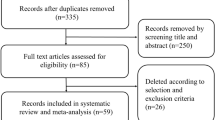
As outlined, the CTRIAL ICORG10/11 is a prospective, multicentre trial which recruited patients from 8 treatment sites in the Republic of Ireland (NCT01722851). Within ICORG 10/11, the primary objective measured was to decipher miRNA targets which could be used to predict treatment response to NAC. The current analysis is a secondary exploratory analysis which uses the data obtained in ICORG 10/11 and correlated these miRNA targets with chemotherapy-induced toxicities. This analysis was performed as per the Standard in Diagnostic Test Accuracy (or STARD) statement to determine the diagnostic test accuracy of miRNAs targets in predicting treatment-induced toxicities [23]. Following a formal power calculation performed by the School of Mathematics, Statistics, and Applied Mathematics at the University of Galway, it was established that 118 patients would require recruitment to accurately address the primary research measure (i.e.: response to NAC), leading to the initial recruitment and inclusion of 120 patients indicated to undergo standard-of-care NAC for primary breast cancer providing informed written consent. Thereafter, 19 of these patients subsequently did not have follow-up information available in relation to treatment-induced toxicities which led to their exclusion from this secondary analysis, leaving 101 patients to be included. Decisions regarding the chemotherapy regimens prescribed were decided based on the professional judgement of the multidisciplinary team in each local tertiary referral centre for breast cancer management. Treatment decisions were made by the breast cancer multidisciplinary team in accordance with internationally accepted standards and guidelines (i.e.: those from the European Society for Medical Oncology and National Comprehensive Cancer Network) [24, 25]. The most common chemotherapy-induced toxicities reported in the seminal TAILORx and RxPONDER prospective, randomised clinical trials were used for comparison in this study [26, 27].
Research ethics
Ethical approval was prospectively obtained from the Galway University Hospitals (C.A.151-February 2008) and University of Galway Clinical Research Institutional boards (C.A.1012-January 2014). In addition, local hospital ethical approval was also obtained from each of the participating centres responsible for patient recruitment.
Participant inclusion and exclusion criteria
Consecutive female patients aged 18 years or older diagnosed and treated for primary breast cancer who were indicated to undergo NAC in accordance with best practice guidelines were considered for inclusion in the current study. All patients had to be capable of providing informed written consent and had to have follow-up data pertaining to treatment-induced toxicities available. Patients were excluded from this study if failed to meet this outlined inclusion criteria.
Tumour profiling and staging
Tumour specimens underwent classification into breast cancer molecular subtypes using the 11th St. Gallen Expert Consensus panel [28], based on the work of Perou et al. [29]. Specimens were analysed as per the 2010 American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) histopathological consensus guidelines for oestrogen (ER) and progesterone (PgR) receptor status using immunohistochemistry [30, 31]. Human epidermal growth factor receptor-2 (HER2) receptor status was delineated using Herceptest™ (DAKO Agilent pathology solutions, Santa Clara, CA, USA), where 3 + was considered to be HER2-positive, with 2 + inconclusive results confirmed using fluorescent in situ hybridisation testing [32, 33]. Appraisal of Ki-67 was performed using MIB1 antibody testing [34, 35].
In brief, luminal cancers (LBC) possessed ER and PgR positivity with HER2 negativity (ER + /PgR + /HER2 −), luminal B-HER2-positive cancers (LBBC-HER2 +) possessed ER + and HER2 positivity with variable PgR expression (ER + /HER2 +), HER2 cancers (HER2 +) possessed ER and PgR negativity with HER2 positivity (ER −/PgR −/HER2 +), and triple negative cancer (TNBC) possessed ER, PgR , and HER negative disease (ER −/PgR −/HER2 −). Tumour staging was performed as per the American Joint Committee on Cancer (AJCC), version 8 guidelines [36].
Venous blood sampling
Venous blood samples from the 101 included patients were collected over a 3-year period (May 2011–April 2014). Whole blood liquid biopsies were collected at five independent timepoints. For this analysis, samples obtained at the time of breast cancer diagnosis (prior to treatment with NAC) were used. Venous blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes and stored at the Organisation of European Cancer Institutes (OECI) approved Comprehensive Cancer Centre Biobank at the Department of Surgery at the University of Galway (UG).
MiRNA expression panel
Based on their previous reported and perceived relevance to breast cancer (i.e.: ontogenetic or tumour suppressor properties), a panel of five miRNAs was selected for evaluation (Let-7a, miR-21, miR-145, miR-155, and miR-195) [37,38,39]. The relevance and rationale for selecting these miRNA targets are illustrated in Table 1. It is important to note that it was initially perceived that miRNAs had only one primary function [37]; however, it is now understood that these biomarkers are multifunctional and may have additional roles in human biology following the administration of cytotoxic chemotherapy that have not previously described (e.g.: immunomodulatory function in bone marrow suppression or inflammation). Therefore, the hypothesis suggesting that these miRNA targets may have credibility in predicting treatment-induced toxicities to NAC is plausible. Two miRNAs (miR-16 and miR-425) were selected and used as validated endogenous controls due to their stability in the circulation of breast cancer patients. These targets were then used to calibrate samples between patients [18, 40]. The relevance of the miRNA selected for inclusion in the panel for this study is outlined in Table 1.
RNA isolation and storage
Total RNA was extracted from whole blood (1 mL) using Trizol (as per the manufacturer’s instructions). RNA concentrations were determined using spectrophotometry (NanoDrop ND-1000 Technologies Inc., Wilmington, DE, USA) [37, 38]. RNA was then transferred to storage tubes and labelled and stored at − 70 °C in the Cancer Biobank at the University of Galway.
Analysis of miRNA expression levels
For each blood sample, miRNAs were relative quantified using polymerase chain reaction (RQ-PCR). TaqMan assays were used, in accordance with the manufacturer’s instructions, for the (RQ-PCR) of the indicated target miRNA (miRNA: Taqman assay ID- miR-195: 000494; miR-155: 002623; miR-145: 002278; miR-21: 000397; Let-7a: 000377; miR-10b: 002218) and the endogenous control (miR-16: 000391; miR-425: 001104), as previously described (TaqMan Fast Universal Master Mix (2X), No AmpErase UNG: Applied biosystems, Foster City, CA, USA, cat:4367846) [11, 40]. Assays were performed using an AB7900HT (Applied Biosystems), using standard conditions as per manufacturer’s instructions. Reactions were commenced with a 10-min incubation at 95 °C before being followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s. MiR-26b was utilised as an inter-assay control which was derived from a breast cancer cell line. These were included on each plate for calibration. To ensure reproducibility to account for outliers, reactions were performed in triplicate (with each individual assay performed using technical triplicates). The threshold standard deviation (SD) for intra-assay and inter-assay replicates was 0.3. The percentage of PCR amplification efficiencies (E) for each assay were calculated using the slope of the semi-log regression plot of cycle threshold vs. log input of cDNA (tenfold dilution series of five points), with the following equation, and a threshold of 10% above or below 100% efficiency was applied: \(E\, = \,\left( {{1}0^{{ - {1}/{\text{slope}}}} \, - \,\,{1}} \right)\, \times \,{1}00\). Moreover, miRNA expression levels were calibrated and normalised using endogenous controls. Thereafter, miRNA expression levels were calculated using QbasePlus© software (Biogazelle, Gent, Belgium) using the geNorm method to ensure the results were calibrated and normalised before being relatively quantified compared to the endogenous controls (miR-16 and miR-425). MiRNA analysis was performed blinded to clinicopathological data.
Definitions of treatment response and toxicity
-
Treatment response to NAC was measured using the Miller-Payne classification system, as outlined initially by Ogston et al. [41].
-
Anaemia; National Cancer Institute Anaemia Scale of grade 2 or worse (i.e.: red blood cell concentrations of 10 g/mL or less) due to chemotherapy-induced bone marrow suppression [42].
-
Neutropenia; the first laboratory evidence of reduced neutrophil count (neutrophil counts less than 2.5 g/mL) in a patient receiving chemotherapy, as per the local hospital guidelines.
-
Neutropenic sepsis; developing pyrexia (temperature 38.0 °C) combined with a neutrophil count of less than 2.5 g/mL while receiving NAC, as per the National Institute for HealthCare Excellence [43].
-
Peripheral neuropathy; chemotherapy-induced neurological symptoms including paraesthesia, paralysis, or neuropathic pain [44].
Test methods and statistical analysis
Regression analyses were performed to correlate miRNA expression with toxicities to NAC, with associated 95% confidence intervals (95%CIs) reported in accordance with the ‘statistical analysis and methods in the published literature’ (or SAMPL guidelines) as previously described by Lang et al.’ [45]. The index test for this analysis was miRNA expression profiles (expressed as continuous data) which were compared to the clinical outcome measure of whether patients developed chemotherapy-induced toxicities (expressed as binary data) [23]. All analyses were two-tailed and statistical significance was defined as P < 0.050. Data were analysed using statistical package for social sciences (SPSS) version 26 (International Business Machines Corporation, Armonk, New York).
Results
Clinicopathological data
In this study, 101 patients were prospectively recruited. The median age at diagnosis was 55.0 years (range: 25.0–76.0) and the mean tumour size was 36.0 mm (range: 10.0–100.0 mm). The majority had nodal involvement (60.4%, n = 61) and 99.0% patients had grade 2/3 disease (n = 100), Overall, 46.5% had luminal (LBC, n = 47), 17.8% had luminal B-HER2 + (LBBC-HER2 + , n = 18), 15.8% (HER2 + , n = 16), and 18.8% had triple negative (TNBC, n = 19).
Neoadjuvant chemotherapy
Overall, 30.7% of patients achieved a pCR (31/101). All 101 patients completed their anticipated NAC regimens (100.0%). Over 50% of patients received doxorubicin and cyclophosphamide followed by paclitaxel (AC-T) (55.5%, n = 56) (Supplementary Material S1). Basic demographic, clinicopathological, and treatment data for the 101 patients included are outlined in Table 2. Exact time interval between index test (i.e.: venous sampling for miRNA expression) and clinical interventions (i.e.: NAC administration) was typically less than 4 weeks.
Chemotherapy-induced toxicities
During treatment with NAC, 33.7% of patients developed symptoms of peripheral neuropathies (n = 34), 26.7% developed nausea and vomiting (n = 27), 5.9% developed myalgia (n = 6), and 11.9% developed mucositis (n = 12). With respect to bone marrow suppression, 28.7% developed neutropenia (n = 29), 59.4% developed lymphopenia (n = 60), and 5.9% developed anaemia (n = 6). Importantly, 2.0% of patients developed neutropenic sepsis (n = 2).
MicroRNA predicting chemotherapy-induced toxicities
Reduced expression of miR-195 predicted patients likely to develop neutropenia during NAC (OR 0.344, 95%CI 0.111–0.990, P = 0.048), while increased expression of miR-10b predicted patients likely to develop anaemia (OR 0.038, 95%CI 0.001–0.910, P = 0.049). Moreover, increased expression of miR-145 predicted patients likely to develop nausea and vomiting during NAC (OR 3.819, 95%CI 1.252–11.652, P = 0.019), while decreased expression of miR-21 predicted patients likely to develop mucositis (OR 0.251, 95%CI 0.090–0.699, P = 0.008). Table 3 illustrates miRNA and their correlation with toxicities to NAC.
Discussion
Previous studies [12], including the primary analysis of the ICORG 10/11 clinical trial [16, 22], have successfully proved that circulating miRNAs have clinical utility in predicting tumour sensitivity to neoadjuvant therapies in patients with breast cancer. At the time of writing, this is the first study to our knowledge which successfully assessed the viability of miRNAs in predicting patients at risk of undesirable treatment-induced toxicities to NAC. The ideology of precision oncology focuses on maximising toxicity to the tumour, while minimising harm to the patient, which sets the foundations for the current analysis. Notwithstanding this, conventional cancer management seems to focus largely on predicting tumour responses to NAC (which has previously been demonstrated the carry a robust survival advantage for the majority) [6, 46, 47], while failing to identify factors indicative chemotherapy-induced toxicity. This study attempts to readjust the focus of translational research studies towards the prediction of patient-specific responses to NAC and to recentre the host (or patient) at the core of treatment paradigm. Therefore, the dogma presented by precision oncology is well captured within the current study, through the provision of novel results illustrating miRNAs as circulatory biomarkers capable of predicting treatment toxicities to NAC. Thus, this study is the first to describe such findings in the oncological literature and will hopefully provide direction for the next generation of translational research studies.
Chemotherapy-induced neutropenia is a major dose-limiting toxicity in clinical oncology and is renowned for placing significant burden upon healthcare economies globally [48]. Identifying patients at risk of this toxicity remains a clinical conundrum. In this study, patients with reduced expression of circulating miR-195 were significantly more likely to develop treatment-induced neutropenia than their counterparts. Aberrant expression of miR-195 has traditionally been recognised as a oncogenic biomarker in breast cancer [38, 49] and there are now emerging data indicating that miRNAs play a regulatory role in circulatory neutrophil proliferation [50]. This is the first report associating miR-195 with myelosuppressive neutropenia, which coincides with the previous data demonstrating miR-195 to be a stress-inducible target with potential immunomodulatory function [51,52,53]. In humans, the miR-195 gene encodes from the reverse strand of the mRNA gene (AK098506), which is responsible for encoding the LOC284112 hypothetical protein, which may have a protective role against bone marrow suppression [54, 55]. While this finding correlating miR-195 expression with treatment-induced neutropenia is one of the significant novelties, we acknowledge that these preliminary results will require further robust interrogation and validation prior to having clinical impact upon therapeutic decision-making in the treatment of cancer patients.
Chemotherapy-induced anaemia has a prevalence of greater than 40% in patients being treated with chemotherapy for breast cancer [56], thus ratifying the necessity for the early detection in this patient cohort. In this study, increased miR-10b was observed in patients who were at an increased risk of developing anaemia during NAC. MiR-10b has been identified to be an oncomir with perceived function in the metastatic cascade in breast cancer [57]; however, previous reports have illustrated the influence of miR-10b on toxicities to chemoradiotherapy in patients with primary glioblastoma, with miR-10b/miR-21 expression correlating with the degree of treatment toxicity [58], an analysis not performed in the current study. Moreover, miR-10b dysregulation has been previously implicated as causative in myelosuppressive disorders [59], making it plausible that increased expression of miR-10b may negatively influence circulatory haemoglobin levels in the post-chemotherapy effect, as illustrated in the current analysis of patients with breast cancer.
In this analysis, increased miR-145 predicted patients likely to develop gastrointestinal dysfunction (i.e.: nausea and vomiting) to NAC, while decreased expression of miR-21 predicted those likely to suffer from the development of mucositis. Inflammation of the gastrointestinal and oral mucosa is a significant negative implication for patients in receipt of chemotherapeutic agents [60, 61]. These provisional results yield promise for the early identification of such patients, which may facilitate early pharmacological prophylaxis. Notwithstanding the inevitability that further scientific assessment is necessary before validating these promising results, these findings may also be used to broaden the horizon to include NAC-induced toxicities within the primary or secondary outcome measures assessed within the next generation of biomarker discovery trials. This is particularly pertinent as current trials often fixate solely upon treatment response as sole analytical endpoints, thus failing to consider the patient-related issues associated with NAC.
This study suffers from several limitations: Firstly, the miRNAs evaluated were included in a predetermined miRNA panel which was decided upon at the time of study design over a decade ago, based on their perceived relevance to breast oncology at that time [37,38,39]. In the time that has elapsed, newer, potentially more relevant miRNAs may have been subsequently discovered. Secondly, this study measures miRNAs from circulation only, failing to evaluate tumour miRNAs from pre-treatment biopsies, meaning tissue yielding crucial genetic information was missed during the tissue acquisition phase of ICORG 10/11. Thirdly, breast cancer is a heterogeneous disease with several biomolecular subtypes with varying treatment algorithms [1], some of which will have evolved since patient recruitment commenced to this study. These factors limit the robustness of these results in present-day contemporary practice. Furthermore, the primary outcome measure of ICORG 10/11 was not powered to provide definitive results among subgroup analyses limiting the robustness of results. Accordingly, the authors have pragmatically not performed subgroup analyses in this secondary exploratory analysis to prevent potential dilution of the robustness of results yielded. Finally, we must highlight these are preliminary results which will inevitably require validation before translation into the contemporary management of breast cancer.
In conclusion, this secondary exploratory analysis is the first from a prospective, multicentre, neoadjuvant translational research trial which successfully assesses the value of measuring circulatory miRNAs to predict patient-specific toxicities to NAC. These results support the persistent ideology that miRNAs may be useful biomarkers with utility in personalising treatment algorithms in accordance with the needs of each patient, while also focusing on patient-specific responses, as well as tumour responses. Thus, this secondary exploratory analysis sets the foundations for the next generation of miRNA clinical trials should be designed to evaluate the use of circulatory miRNAs to predict the treatment-induced toxicities to NAC to ensure patient monitoring is individualised for prospective patients.
Data availability
Data will be made available upon reasonable request from the senior author, MJK.
References
Davey MG, Kerin MJ (2023) Molecular profiling in contemporary breast cancer management. Br J Surg 110:xnad017
Burstein HJ, Curigliano G, Thürlimann B et al (2021) Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 32(10):1216–1235
Asselain B et al (2018) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19(1):27–39
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97(3):188–194
Schott AF, Hayes DF (2012) Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol 30(15):1747–1749
Davey MG, Browne F, Miller N et al (2022) Pathological complete response as a surrogate to improved survival in human epidermal growth factor receptor-2-positive breast cancer: systematic review and meta-analysis. BJS Open. 6(3):zrac028
Wu XYC, Wang X, Cai R, Yang J, Yu X, Zhou Y, Shen L, Zhu Y, Liu X (2021) The efficacy and toxicity of neoadjuvant chemotherapy regimens of epirubicin plus cyclophosphamide followed by docetaxel or paclitaxel in female breast cancer patients. Cancer Manage Res 13:1517–1527
Amjad MT, Chidharla A, Kasi A (2022) Cancer chemotherapy. StatPearls Publishing, Treasure Island
Behranvand N, Nasri F, Zolfaghari Emameh R et al (2022) Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother 71(3):507–526
Friese CR, Harrison JM, Janz NK et al (2017) Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer 123(11):1925–1934
Davey MG, Lowery AJ, Miller N et al (2021) MicroRNA expression profiles and breast cancer chemotherapy. Int J Mol Sci 22(19):10812
Davey MG, Davies M, Lowery AJ et al (2021) The role of microRNA as clinical biomarkers for breast cancer surgery and treatment. Int J Mol Sci 22(15):8290
Richard V, Davey MG, Annuk H et al (2021) MicroRNAs in molecular classification and pathogenesis of breast tumors. Cancers (Basel). 13(21):5332
O’Brien J, Hayder H, Zayed Y et al (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. https://doi.org/10.3389/fendo.2018.00402
Mitchell PS, Parkin RK, Kroh EM et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 105(30):10513–10518
Davey MG, Casey MC, McGuire A et al (2022) Evaluating the role of circulating microRNAs to aid therapeutic decision making for neoadjuvant chemotherapy in breast cancer: a prospective. Multicenter Clinical Trial Ann Surg 276(5):905–912
Davey MG, Feeney G, Annuk H et al (2022) MicroRNA expression profiling predicts nodal status and disease recurrence in patients treated with curative intent for colorectal cancer. Cancers (Basel). 14(9):2109
Davey MG, McGuire A, Casey MC et al (2023) Evaluating the role of circulating microRNAs in predicting long-term survival outcomes in breast cancer: a prospective, multicenter clinical trial. J Am Coll Surg 236(2):317–327
Pellegrini L, Sileno S, D’Agostino M et al (2020) MicroRNAs in cancer treatment-induced cardiotoxicity. Cancers 12(3):704
Brown C, Mantzaris M, Nicolaou E et al (2022) A systematic review of miRNAs as biomarkers for chemotherapy-induced cardiotoxicity in breast cancer patients reveals potentially clinically informative panels as well as key challenges in miRNA research. Cardio-Oncology 8(1):16
Wan G, Lim QE, Too HP (2010) High-performance quantification of mature microRNAs by real-time RT-PCR using deoxyuridine-incorporated oligonucleotides and hemi-nested primers. RNA 16(7):1436–1445
McGuire A, Casey MC, Waldron RM et al (2020) Prospective assessment of systemic microRNAs as markers of response to neoadjuvant chemotherapy in breast cancer. Cancers (Basel). 12(7):1820
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351:h5527
Gradishar WJ, Moran MS, Abraham J et al (2022) Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(6):691–722
Senkus E, Kyriakides S, Penault-Llorca F et al (2013) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(6):vi7–vi23
Sparano JA, Gray RJ, Makower DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121
Kalinsky K, Barlow WE, Gralow JR et al (2021) 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 385(25):2336–2347
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22(8):1736–1747
Perou CM, Sørlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Allred DC (2010) Issues and updates: evaluating estrogen receptor-alpha, progesterone receptor, and HER2 in breast cancer. Mod Pathol 23(Suppl 2):S52–S59
Mahlow J, Goold EA, Jedrzkiewicz J et al (2021) What to expect from the new ASCO/CAP guideline recommendations for hormone receptor testing in breast cancer: a national reference laboratory experience. Appl Immunohistochem Mol Morphol 29(4):245–250
Moelans CB, de Weger RA, Van der Wall E et al (2011) Current technologies for HER2 testing in breast cancer. Crit Rev Oncol Hematol 80(3):380–392
Kostopoulou E, Vageli D, Kaisaridou D et al (2007) Comparative evaluation of non-informative HER-2 immunoreactions (2+) in breast carcinomas with FISH, CISH and QRT-PCR. Breast 16(6):615–624
Dowsett M, Nielsen TO, A’Hern R et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in breast cancer working group. J Natl Cancer Inst 103(22):1656–1664
Davey MG, Hynes SO, Kerin MJ et al (2021) Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers (Basel). 13(17):4455
Amin MB, Greene FL, Edge SB et al (2017) The eight edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67(2):93–99
Heneghan HM, Miller N, Lowery AJ et al (2010) Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg 251(3):499–505
Heneghan HM, Miller N, Kelly R et al (2010) Systemic miRNA-195 differentiates breast cancer from other malignancies and is a potential biomarker for detecting noninvasive and early stage disease. Oncologist 15(7):673–682
Lowery AJ, Miller N, Devaney A et al (2009) MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res 11(3):R27
McDermott AM, Kerin MJ, Miller N (2013) Identification and validation of miRNAs as endogenous controls for RQ-PCR in blood specimens for breast cancer studies. PLoS ONE 8(12):e83718
Ogston KN, Miller ID, Payne S et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12(5):320–327
Network, NCC (2018) NCCN clinical practice guidelines in oncology. Cancer and chemotherapy induced anemia. Version 2. https://www.nccn.org
NICE (2012) Neutropenic sepsis: prevention and management in people with cancer 2012 Clinical guideline (CG151). https://www.nice.org.uk/guidance/cg151.
Li T, Mizrahi D, Goldstein D et al (2021) Chemotherapy and peripheral neuropathy. Neurol Sci 42(10):4109–4121
Lang TA, Altman DG (2015) Basic statistical reporting for articles published in biomedical journals: the “statistical analyses and methods in the published literature” or the SAMPL guidelines. Int J Nurs Stud 52(1):5–9
Spring LM, Fell G, Arfe A et al (2020) Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 26(12):2838–2848
Davey MG, Kerin E, O’Flaherty C et al (2021) Clinicopathological response to neoadjuvant therapies and pathological complete response as a biomarker of survival in human epidermal growth factor receptor-2 enriched breast cancer—a retrospective cohort study. Breast 59:67–75
Boccia R, Glaspy J, Crawford J et al (2022) Chemotherapy-induced neutropenia and febrile neutropenia in the US: a beast of burden that needs to be tamed? Oncologist 27(8):625–636
McAnena P, Tanriverdi K, Curran C et al (2019) Circulating microRNAs miR-331 and miR-195 differentiate local luminal a from metastatic breast cancer. BMC Cancer 19(1):436
Larsen MT, Hother C, Häger M et al (2013) MicroRNA profiling in human neutrophils during bone marrow granulopoiesis and in vivo exudation. PLoS ONE 8(3):e58454
Darden DB, Stortz JA, Hollen MK et al (2020) Identification of unique mRNA and miRNA expression patterns in bone marrow hematopoietic stem and progenitor cells after trauma in older adults. Front Immunol 11:1289
Almeida MI, Silva AM, Vasconcelos DM et al (2016) miR-195 in human primary mesenchymal stromal/stem cells regulates proliferation, osteogenesis and paracrine effect on angiogenesis. Oncotarget 7(1):7–22
Yu W, Liang X, Li X et al (2018) MicroRNA-195: a review of its role in cancers. Onco Targets Ther 11:7109–7123
Flavin RJ, Smyth PC, Laios A et al (2009) Potentially important microRNA cluster on chromosome 17p13.1 in primary peritoneal carcinoma. Modern Pathol 22(2):197–205
He JF, Luo YM, Wan XH et al (2011) Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle, and apoptosis. J Biochem Mol Toxicol 25(6):404–408
Sharma P, Georgy JT, Andrews AG et al (2022) Anemia requiring transfusion in breast cancer patients on dose-dense chemotherapy: prevalence, risk factors, cost and effect on disease outcome. Support Care Cancer 30(6):5519–5526
Ma L (2010) Role of miR-10b in breast cancer metastasis. Breast Cancer Res 12(5):210
Stepanović A, Nikitović M, Stanojković TP et al (2022) Association between microRNAs 10b/21/34a and acute toxicity in glioblastoma patients treated with radiotherapy and temozolomide. Sci Rep 12(1):7505
Fang J, Varney M, Starczynowski DT (2012) Implication of microRNAs in the pathogenesis of MDS. Curr Pharm Des 18(22):3170–3179
Barkokebas A, Silva IH, de Andrade SC et al (2015) Impact of oral mucositis on oral-health-related quality of life of patients diagnosed with cancer. J Oral Pathol Med 44(9):746–751
Yeo W, Mo FKF, Yip CCH et al (2021) Quality of life associated with nausea and vomiting from anthracycline-based chemotherapy: a pooled data analysis from three prospective trials. Oncologist 26(12):e2288–e2296
Acknowledgements
The study was supported by Clinical Trials Ireland (formerly the All-Ireland Cooperative Oncology Research Group; ICORG) and the National Breast Cancer Research Institute (Ireland).
Funding
Open Access funding provided by the IReL Consortium. Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.M.H, N.M, and M.J.K: circulating miR-195 as a biomarker patent. All miRNA were measured on blinded samples and the unblinded analysis was performed by independent study statisticians. All other authors declare no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Davey, M.G., Abbas, R., Kerin, E.P. et al. Circulating microRNAs can predict chemotherapy-induced toxicities in patients being treated for primary breast cancer. Breast Cancer Res Treat 202, 73–81 (2023). https://doi.org/10.1007/s10549-023-07033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07033-8