Abstract
Purpose
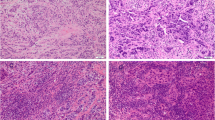
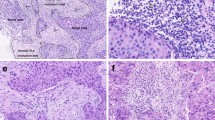
The tumor-stroma ratio (TSR) is a common histological parameter that measures stromal abundance and is prognostic in breast cancer (BC). However, more evidence is needed on the predictive value of the TSR for the pathological complete response (pCR) to neoadjuvant chemotherapy (NAC). The purpose of this study was to determine the importance of the TSR in predicting pCR in NAC settings.
Method
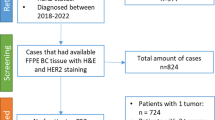
We evaluated the TSR on pretreatment biopsies of 912 BC patients from four independent Chinese hospitals and investigated the potential value of the TSR for predicting pCR. Meanwhile, stromal tumor-infiltrating lymphocytes (sTILs) were assessed, and we evaluated the predictive value of the combination of sTILs and TSR (TSRILs).
Results
Patients with low stroma showed a higher pCR rate than those with high stroma among the four independent hospitals, and in multivariate analysis, the TSR was proven to be an independent predictor for pCR to NAC with an odds ratio of 1.945 (95% CI 1.230–3.075, P = 0.004). Moreover, we found that TSRILs could improve the area under the curve (AUC) for predicting pCR from 0.750 to 0.785 (P = 0.039); especially in HER2-negative BCs, the inclusion of TSRILs increased the AUC from 0.801 to 0.835 in the discovery dataset (P = 0.048) and 0.734 to 0.801 in the validation dataset (P = 0.003).
Conclusion
TSR and sTILs can be easily measured in pathological routines and provide predictive information without additional cost; with more evidence from clinical trials, TSRILs could be a candidate to better stratify patients in NAC settings.





Similar content being viewed by others
Data availability
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33
Derks MGM, Van De Velde CJH (2018) Neoadjuvant chemotherapy in breast cancer: more than just downsizing. Lancet Oncol 19(1):2–3
Von Minckwitz G, Blohmer JU, Costa SD et al (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31(29):3623–3630
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Goorts B, Van Nijnatten TJ, De Munck L et al (2017) Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat 163(1):83–91
Denkert C, Von Minckwitz G, Darb-Esfahani S et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50
Ali HR, Dariush A, Thomas J et al (2017) Lymphocyte density determined by computational pathology validated as a predictor of response to neoadjuvant chemotherapy in breast cancer: secondary analysis of the ARTemis trial. Ann Oncol 28(8):1832–1835
Denkert C, Loibl S, Noske A et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113
Carey LA, Berry DA, Cirrincione CT et al (2016) Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol 34(6):542–549
Abdel-Fatah TMA, Agarwal D, Liu DX et al (2016) SPAG5 as a prognostic biomarker and chemotherapy sensitivity predictor in breast cancer: a retrospective, integrated genomic, transcriptomic, and protein analysis. Lancet Oncol 17(7):1004–1018
Pineda B, Diaz-Lagares A, Pérez-Fidalgo JA et al (2019) A two-gene epigenetic signature for the prediction of response to neoadjuvant chemotherapy in triple-negative breast cancer patients. Clin Epigenet 11(1):33
Alba E, Rueda OM, Lluch A et al (2018) Integrative cluster classification to predict pathological complete response to neoadjuvant chemotherapy in early breast cancer. J Clin Oncol 36(15_suppl):579
Farmer P, Bonnefoi H, Anderle P et al (2009) A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 15(1):68–74
Lee H, Lee DE, Park S et al (2019) Predicting response to neoadjuvant chemotherapy in patients with breast cancer: combined statistical modeling using clinicopathological factors and FDG PET/CT texture parameters. Clin Nucl Med 44(1):21–29
Li F, Yang Y, Wei Y et al (2021) Deep learning-based predictive biomarker of pathological complete response to neoadjuvant chemotherapy from histological images in breast cancer. J Transl Med 19(1):348
Lips EH, Mulder L, De Ronde JJ et al (2013) Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response. Breast Cancer Res Treat 140(1):63–71
Alba E, Lluch A, Ribelles N et al (2016) High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. Oncologist 21(6):778
Hale MD, Hayden JD, Grabsch HI (2013) Tumour-microenvironment interactions: role of tumour stroma and proteins produced by cancer-associated fibroblasts in chemotherapy response. Cell Oncol (Dordr) 36(2):95–112
Dittmer J, Leyh B (2015) The impact of tumor stroma on drug response in breast cancer. Semin Cancer Biol 31:3–15
Ireland LV, Mielgo A (2018) Macrophages and fibroblasts, key players in cancer chemoresistance. Front Cell Dev Biol 6:131
Su S, Chen J, Yao H et al (2018) CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172(4):841–56.e16
Xing H, Weng D, Chen G et al (2008) Activation of fibronectin/PI-3K/Akt2 leads to chemoresistance to docetaxel by regulating survivin protein expression in ovarian and breast cancer cells. Cancer Lett 261(1):108–119
Mesker WE, Junggeburt JM, Szuhai K et al (2007) The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 29(5):387–398
De Kruijf EM, Van Nes JG, Van De Velde CJ et al (2011) Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 125(3):687–696
Dekker TJ, Van De Velde CJ, Van Pelt GW et al (2013) Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res Treat 139(2):371–379
Roeke T, Sobral-Leite M, Dekker TJA et al (2017) The prognostic value of the tumour-stroma ratio in primary operable invasive cancer of the breast: a validation study. Breast Cancer Res Treat 166(2):435–445
Vangangelt KMH, Tollenaar LSA, Van Pelt GW et al (2018) The prognostic value of tumor-stroma ratio in tumor-positive axillary lymph nodes of breast cancer patients. Int J Cancer 143(12):3194–3200
Vangangelt KMH, Van Pelt GW, Engels CC et al (2018) Prognostic value of tumor-stroma ratio combined with the immune status of tumors in invasive breast carcinoma. Breast Cancer Res Treat 168(3):601–612
Vangangelt KMH, Green AR, Heemskerk IMF et al (2020) The prognostic value of the tumor-stroma ratio is most discriminative in patients with grade III or triple-negative breast cancer. Int J Cancer 146(8):2296–2304
Kramer CJH, Vangangelt KMH, Van Pelt GW et al (2019) The prognostic value of tumour-stroma ratio in primary breast cancer with special attention to triple-negative tumours: a review. Breast Cancer Res Treat 173(1):55–64
Moorman AM, Vink R, Heijmans HJ et al (2012) The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol 38(4):307–313
Yan D, Ju X, Luo B et al (2022) Tumour stroma ratio is a potential predictor for 5-year disease-free survival in breast cancer. BMC Cancer 22(1):1082
Hagenaars SC, De Groot S, Cohen D et al (2021) Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer. Int J Cancer 149(5):1181–1188
Mallya V, Singh V, Kaur N et al (2020) Does tumor stroma ratio of breast cancer trucut biopsy determine response to neoadjuvant therapy? Indian J Pathol Microbiol 63(Supplement):S113–S116
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271
Hagenaars SC, Vangangelt KMH, Van Pelt GW et al (2022) Standardization of the tumor-stroma ratio scoring method for breast cancer research. Breast Cancer Res Treat 193(3):545–553
Le MK, Odate T, Kawai M et al (2023) Investigating the role of core needle biopsy in evaluating tumor-stroma ratio (TSR) of invasive breast cancer: a retrospective study. Breast Cancer Res Treat 197(1):113–121
Rindi G, Klimstra DS, Abedi-Ardekani B et al (2018) A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 31(12):1770–1786
Sonnenblick A, Salmon-Divon M, Salgado R et al (2020) Reactive stroma and trastuzumab resistance in HER2-positive early breast cancer. Int J Cancer 147(1):266–276
Rybinska I, Sandri M, Bianchi F et al (2020) Extracellular matrix features discriminate aggressive HER2-positive breast cancer patients who benefit from trastuzumab treatment. Cells 9(2):434
Fernández-Nogueira P, Mancino M, Fuster G et al (2020) Tumor-associated fibroblasts promote HER2-targeted therapy resistance through FGFR2 activation. Clin Cancer Res 26(6):1432–1448
Li F, Yang Y, Wei Y et al (2022) Predicting neoadjuvant chemotherapy benefit using deep learning from stromal histology in breast cancer. NPJ Breast Cancer 8(1):124
Gujam FJ, Edwards J, Mohammed ZM et al (2014) The relationship between the tumour stroma percentage, clinicopathological characteristics and outcome in patients with operable ductal breast cancer. Br J Cancer 111(1):157–165
Funding
The study was supported by the 1·3·5 project for disciplines of excellence (ZYGD18012) and the Technological Innovation Project of Chengdu New Industrial Technology Research Institute (2017-CY02–00026-GX).
Author information
Authors and Affiliations
Contributions
FL, HC, and HB designed this study. YW, YZ, JF, XX, and FL contributed to the patient recruitment from four hospitals. XL and FL performed the evaluation of pathological parameters. FL and HC analyzed the data and wrote the draft. HB and HC edited the draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Our study was approved by the ethical committee of West China Hospital, Sichuan University (No.764 in 2021), and abided with the Declaration of Helsinki before using tissue samples for scientific researches purpose only. The written informed consent was waived by the ethical committee for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, F., Chen, H., Lu, X. et al. Combining the tumor-stroma ratio with tumor-infiltrating lymphocytes improves the prediction of pathological complete response in breast cancer patients. Breast Cancer Res Treat 202, 173–183 (2023). https://doi.org/10.1007/s10549-023-07026-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07026-7