Abstract
Purpose
To determine whether six cycles of FEC3-D3 has a comparable efficacy to eight of AC4-D4.
Methods
The enrolled patients (pts) were clinically diagnosed with stage II or III breast cancer. The primary endpoint was a pathologic complete response (pCR), and the secondary endpoints were 3 year disease-free survival (3Y DFS), toxicities, and health-related quality of life (HRQoL). We calculated that 252 pts were needed in each treatment group to enable the detection of non-inferiority (non-inferiority margin of 10%).
Results
In terms of ITT analysis, 248 pts were finally enrolled. The 218 pts who completed the surgery were included in the current analysis. The baseline characteristics of these subjects were well balanced between the two arms. By ITT analysis, pCR was achieved in 15/121 (12.4%) pts in the FEC3-D3 arm and 18/126 (14.3%) in the AC4-D4 arm. With a median follow up of 64.1 months, the 3Y DFS was comparable between the two arms (75.8% in FEC3-D3 vs. 75.6% in AC4-D4). The most common adverse event (AE) was Grade 3/4 neutropenia, which arose in 27/126 (21.4%) AC4-D4 arm pts vs 23/121 (19.0%) FEC3-D3 arm cases. The primary HRQoL domains were similar between the two groups (FACT-B scores at baseline, P = 0.35; at the midpoint of NACT, P = 0.20; at the completion of NACT, P = 0.44).
Conclusion
Six cycles of FEC3-D3 could be an alternative to eight of AC4-D4. Trial registration ClinicalTrials.gov NCT02001506. Registered December 5,2013. https://clinicaltrials.gov/ct2/show/NCT02001506
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The previous national surgical adjuvant breast and bowel project (NSABP) B18 study [1] demonstrated no significant difference in overall survival (OS) or disease free survival (DFS) between adjuvant and neoadjuvant chemotherapy, also confirmed in many subsequent studies [2,3,4]. Although there has been no reported survival gain with neoadjuvant chemotherapy, it has been used to reduce the extent of local therapy or reduce delays in initiating therapy [1, 5,6,7]. In addition, some studies have confirmed that achieving a pathologic complete response (pCR) after neoadjuvant chemotherapy is significantly helpful in predicting long term survival outcomes [1,2,3,4]. Neoadjuvant chemotherapy has thus become a standard of care that can be considered for locally advanced breast cancer.
The previous randomized NSABP-B27 study reported a 90% overall clinical response rate after four cycles of AC followed by four cycles of docetaxel [8]. Three cycles of FEC (fluorouracil, epirubicin, and cyclophosphamide) followed by three cycles of docetaxel, compared to six cycles of FEC, in an adjuvant setting have also demonstrated a survival benefit [9]. Three cycles of FEC followed by three cycles of docetaxel (FEC3-D3) was a popular neoadjuvant chemotherapy regimen in europe when this study was designed. Six rather than eight cycles have an advantage in terms of a shorter treatment duration with lower toxicities and a higher compliance unless efficacy is compromised. Docetaxel can also be used at a dose of 75 mg/m2 in each cycle considering that the higher 100 mg/m2 dose showed no clinical benefit from the higher toxicity in previous studies [10, 11], and would be more feasible in a neoadjuvant setting in terms of a reduced toxicity and improved tolerance. However, there have been limited reports to date on whether efficacy is maintained, or quality of life (QoL) is reduced, when the number of treatment cycles is reduced. In our present study, we compared the degree of efficacy and QoL over the course of the neoadjuvant chemotherapy intervention in patients who underwent AC4-D4 or FEC3-D3 as a preoperative chemotherapy regiment for stage II or III breast cancer.
Patients and methods
Study design and objectives
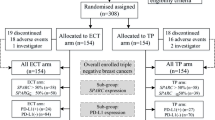
This was a randomized, prospective, parallel group, comparative phase 3 study conducted at Asan Medical Center, Seoul, Korea. The patient allocation is outlined in Fig. 1. The primary outcome was pCR from a node-positive breast cancer treated with an FEC3-D3 or AC4-D4 neo-adjuvant chemotherapy regimen. Secondary outcomes included 3-year disease free survival (3Y DFS), quality of life (QoL), and the correlation between Ki-67 expression and pCR, which was defined as no evidence of invasive cancer in the breast or lymph nodes. Detailed descriptions of the study methodology and eligibility criteria are provided in the Supplementary Information.
Procedures
Three cycles of FEC followed by three cycles of docetaxel (FEC3-D3) were administered by intravenous injection every 3 weeks using the following dosages: 5-fluorouracil, 500 mg/m2; epirubicin, 100 mg/m2; cyclophosphamide, 500 mg/m2; and docetaxel, 75 mg/m2. Four cycles of AC followed by 4 cycles of docetaxel (AC4-D4) were also administered by intravenous injection every 3 weeks as follows: adriamycin, 60 mg/m2; cyclophosphamide, 600 mg/m2; and docetaxel, 75 mg/m2. Mammography and breast ultrasounds were done at the midpoint (after the three cycles of FEC in arm A and four cycles of AC in arm B) and at the completion of the chemotherapy. Breast magnetic resonance (MR) was performed at baseline and before surgery. Surgery was undertaken within 6 weeks of the last round of chemotherapy. The administration of adjuvant chemotherapy, hormonal therapy and/or trastuzumab, and postoperative radiation was at the discretion of the treating physician. The relative dose intensity (RDI) is the ratio of the actual dose intensity of chemotherapy delivered to the standard recommended dose intensity [12].
Response and toxicity assessments
Response assessments were done using RECIST version 1.1. Adverse events (AEs) were evaluated every 3 weeks (±1 week) using Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Patients who had received at least one cycle of chemotherapy were included in toxicity assessment. A QoL assessment was conducted at the midpoint and at the completion of the chemotherapy using Functional Assessment of Cancer Therapy-Breast (FACT-B) version 4.0 [13].
Follow up methods after the surgery
Post-op follow ups were done every 3 to 6 months for the first 2 years and then every 6 months for up to 5 years and included a physical examination, CBC, laboratory chemistry tests, and an annual mammogram with or without breast sonography. After then, follow ups were done annually.
Statistical analysis
With a two-sided type Ι error of 0.05 and a power of 80%, we calculated that 252 pts were needed in each treatment group to enable the detection of non-inferiority of neoadjuvant FEC3-D3 compared to AC4-D4 with a pCR rate of 20% (non-inferiority margin of 10%). Considering a dropout rate of 10%, and 280 pts in each arm, a total of 560 patients per arm would be enrolled. Pts were randomized using the stratified block randomization method with the hormone receptor and HER2 expression status included as the stratification factors. The sample size was amended due to slower enrolment and competing trials. The revised statistical procedure was that all parameters would be analyzed using descriptive statistics. Disease free survival was calculated with the Kaplan–Meier method. Categorical variables were expressed as proportions and continuous variables as the mean ± SD. The Mann–Whitney U-test was used to compare differences between the treatment arms. The Friedman test was used to detect repeated measurement difference. Statistical analysis was done using SPSS version 23.0 (IBM Corp, Armonk, NY), and statistical significance was defined as a P value less than 0.05. The cut off value of the Ki-67 labeling index was determined by the AUC curve based on the values of the highest sensitivity and specificity. The intention to treat (ITT) population was defined as all the patients who were randomized, excluding those who failed the screening. The per-protocol population was defined as patients who completed the study.
Results
Baseline characteristics of the total cohort
In this present study series, 248 patients diagnosed with stage II or III breast cancer between November 2012 and December 2015 were enrolled. These cases were randomly assigned (1:1) to an FEC3-D3 (n = 121, 48.9%) or AC4-D4 (n = 126, 51.1%) treatment arm. Subsequent to this enrollment, one patient was found to be ineligible for screening; 10 discontinued treatment due to progressive disease (7 in the AC4-D4 arm and 3 in the FEC3-D3 arm), 16 patients withdrew consent to participate (13 in the AC4-D4 arm and 3 in the FEC3-D3 arm), and three patients were unable to complete the study (2 in the AC4-D4 arm due to exceeding the dose delay limit of 9 weeks and grade 3 peripheral neuropathy, and one patient in the FEC3-D3 arm due to a loss of consciousness of unknown etiology). Ten out of the 247 patients (4.0%) experienced progression during the neoadjuvant chemotherapy. Two of them were unable to undergo surgery because they had a distant metastasis. The 218 remaining patients receiving surgery were included in our per-protocol analysis. The baseline characteristics were well balanced in terms of median age (49 vs 47), percentage of luminal type cases (66.1% vs 69.1%), and percentage of triple negative breast cancers (20.7% vs. 19.0%) between the FEC3-D3 (n = 121) and AC4-D4 (n = 126) arms. Clinical T2 (57.5% vs. 62.6%) and N1 (63.3% vs. 64.3%) stage tumors were also predominant in both arms (Table 1).
Pathologic complete response outcomes and correlations with the baseline Ki-67 labeling index
By intention-to-treat (ITT) analysis, pCR was achieved in 15/121 (12.4%) patients in the FEC3-D3 arm and 18/126 (14.3%) patients in the AC4-D4 arm. In the FEC3-D3 arm, 92/114 patients achieved a clinical response [4 complete responses (CR) and 88 partial responses (PR)] and among these cases, 15 patients (12.4%) achieved pCR. In the AC4-D4 arm, 95/104 patients achieved a clinical response (6 CR and 89 PR), among which 18 patients (14.3%) achieved pCR (Table 2). In terms of pCR, eight cycles were numerically slightly higher than six cycles even when analyzed by subtype (Table S2 in the Supplementary material). When different cut-offs for Ki 67 were assessed in the luminal B subtype, a Ki 67≥55% was associated with a higher pCR rate.
Three-year disease-free survival outcomes
With a median follow up of 64.1 months, the 3Y DFS (75.8% in FEC3-D3 vs. 75.6% in AC4-D4) was comparable between the two arms Fig. 2A. Forest plots of the 3Y DFS for the subgroups in the ITT analysis are shown in Fig. 3. In the subgroup analysis, there was no favorable regimen between FEC3-D3 and AC4-D4. Univariate and multivariate analyses of the associations between the clinicopathologic factors and 3Y DFS are summarized in Table S1 in the Supplementary material. For the 3Y DFS, ≥ 55% of the baseline Ki-67 labeling index with luminal type (HR 2.1, 95% CI, 1.04–4.25), and ≥ 4 lymph node metastases at surgery (HR 1.9, 95% CI, 1.07–3.51) seemed to correlate with the 3Y DFS.
Kaplan–Meier plots of disease free survival outcomes. (a) Intention to treat. (b) Per protocol. AC4-D4 adriamycin, and cyclophosphamide (4 cycles) followed by docetaxel (4 cycles), CI confidence interval, FEC3-D3 fluorouracil, epirubicin, and cyclophosphamide (3 cycles) followed by docetaxel (3 cycles)
Subgroup analysis of 3 year disease free survival (3Y DFS) outcomes in the intention to treat population. AC4-D4 adriamycin, and cyclophosphamide (4 cycles) followed by docetaxel (4 cycles), CI confidence interval, FEC3-D3 fluorouracil, epirubicin, and cyclophosphamide (3 cycles) followed by docetaxel (3 cycles), HER2 human epidermal growth factor receptor 2, HR hazard ratio, TNBC triple negative breast cancer
Toxicity and QoL assessments
The most common AE was a Grade 3/4 neutropenia [27/126 (21.4%) patients in the AC4-D4 arm vs. 23/121 (19.0%) patients in the FEC3-D3 arm]. The most common Grade 3/4 non-hematologic AE was hyperglycemia (4.0%). A dose modification was made in 25/121 (20.7%) patients in the FEC3-D3 arm and 37/126 (29.4%) in the AC4-D4 arm (Table 3). The number of patients who completed chemotherapy were 114 out of 121 in the FEC3-D3 arm and 104 out of 126 in the AC4-D4 arm. A 20% dose modification was performed on 22 of 114 patients in the FEC3 group comprising three patients from cycle 1 and 19 patients from cycle 2. The relative dose intensity (RDI) for three cycles of FEC was 95.5%. Dose modification was performed in 6 of 114 patients in the D3 group. The RDI for three cycles of docetaxel was 99.2%. Dose modification was performed for 16 of 104 patients in the AC4 group. The RDI for 4 cycles of AC was 97.0%. A 20% dose modification was performed for 26 of 104 patients who had completed chemotherapy in the D4 group. The RDI for four cycles of docetaxel was 97.1%.
The QoL scores determined by FACT-B version 4 are listed in Table 4. The mean QoL values at baseline were 102.39, (standard deviation (SD), 17.50) vs.100.74 (SD, 16.72), at the midpoint of neoadjuvant chemotherapy were 85.24 (SD, 36.80) vs. 79.28 (SD, 38.62), and at the completion of chemotherapy were 75.71 (SD, 39.53) vs.70.73 (SD, 42.25) in the FEC3-D3 vs. AC4-D4 arms, respectively. In the FACT-B subgroups, emotional wellbeing (EWB) showed the lowest scores in both groups at baseline [FEC3-D3, 16.71 (SD, 4.81) vs. AC4-D4, 15.89 (SD, 4.79)]. Social wellbeing (SWB) had the lowest score in the FEC3-D3 arm [15.23 (SD,8.16)], whereas functional wellbeing (FWB) displayed the lowest score in the AC4-D4 arm [13.81 (SD, 7.61)], at the midpoint of the neoadjuvant chemotherapy. FWB was the lowest in both groups at the completion of chemotherapy [FEC3-D3, 12.83 (SD, 7.76) vs. AC4-D4, 12.72 (SD, 8.52)].
Discussion
FEC 3 followed by docetaxel 3 had been one of representative preoperative/adjuvant chemotherapy regimens specified in the NCCN guidelines up to 2017.[14,15,16,17,18]. Notably however, the FEC regimen was excluded from the NCCN guidelines after the NSABP-B36 trial [19]. In that study, six cycles of an FEC regimen did not show a superior efficacy to 4 AC cycles but did show a higher toxicity. Since the NSABP-B27 report, the AC4-D4 regimen has become widely used. However, the eight cycles of treatment in this protocol requires 6 months to complete, and there have been concerns regarding the reduction in patient compliance that is commonly related to a longer treatment duration. In addition, there is a reported QoL decrease due to increased exposure to anthracycline and taxane in the AC4-D4 regimen. The dose dense regimen has recently become widely used in the United states and Europe. There has also been a recent study demonstrating the superiority of the dose dense regimen [20]. However, at the beginning of our study in 2012, there was only a phase 2 study for dose-dense regimen and no randomized phase 3 trial. Also, in 2012, the dose dense regimen was not found to be superior by meta-analysis and was not available in daily clinical practice. Notably in this regard, the pCR and 3Y DFS showed no significant difference between our current study and two prior reports [21, 22], which investigated dose dense regimens as an NACT. Also, there was no significant difference in the 3Y DFS between a previous study[23] that used a dose dense regimen as post operative therapy and our current investigation. Prophylactic pegylated G-CSF (peg G-CSF) is required for a dose dense regimen and was not available as a primary prophylaxis at the beginning of this study.
The pCR rate in our present study series was low compared to the 26.1% level reported in the NSABP-B27 [8]. The pCR rate is known to be higher after neoadjuvant chemotherapy in the absence of HER2, estrogen receptor (ER) positivity and a lack of lymph node metastasis [24,25,26,27]. The different pCR rate between our current investigation and the NSABP B27 may have been due to the greater number of lymph node metastases [247/247 (100%) vs. 244/805 (30.3%)] and also the higher ER positivity [167/247 (67.6%) vs. 319/805 (39.6%) in Neo-Shorter vs. NSABP-B27]. In the NSABP-B27 study, the pCR rate in the AC followed by taxane treatment group with ER positivity was 14.1%, comparable to the 17.3% rate found in our present series. In addition, our observed pCR rate was low compared to that of a previous Indian study with a similar design concept [28]. The difference in the pCR rate between our present study and that prior Indian report may also have been due to differences in the proportion of triple negative breast cancers (TNBCs) and HER2-positive tumors, even though they have a similar clinical stage (Neo-Shorter vs. India, 32.4% vs. 49%). Also, in the prior study cohort from India, unlike our present series, HER2 2 + was considered to be negative without further HER2 in situ hybridization being conducted, which may have affected the findings.
Similar to previous studies, the higher Ki67 level among patients with the luminal type breast tumors in our present cohort was associated with a higher pCR. There was no significant correlation found between Ki-67 and the pCR rate in previous TNBC studies, or among these cases in our present study, and similar findings were also demonstrated in the prior Gepar TRIO trial [29]. However, there was a significant correlation found in our current analyses, in the luminal type, between the pCR and a Ki 67 index that was equal to or more than 55%.
Our current multivariate analysis revealed that a ≥ 55% baseline Ki-67 labeling index with luminal (HR 2.1, 95% CI,1.04–4.25), and ≥ 4 lymph node metastases at surgery (HR 1.9, 95% CI, 1.07–3.51) seemed to be correlated with the 3Y DFS outcome. A previous study found that an age > 50, higher T and N clinical stage, or tumor size > 5 cm were independent risk factors for distant metastasis in TNBC [30]. Additionally, in a previous meta-analysis study by Salvo et al. of hormone receptor-positive and HER2-negative breast cancers, it was confirmed that lymph-node positivity was an important factor for recurrence [31]. Our present results were consistent with the previously reported criteria for high-risk recurrence in TNBCs or hormone receptor-positive breast cancers. In terms of the 3Y DFS, the difference between our current study findings and those of the PACS01 trial appears be an effect of the inclusion ratio of stage I (neo-shorter: stage I, 0%, 3Y DFS, 77.0% vs. PACS01: stage I, 10.4%, 3Y DFS, 84.5%). Similarly, the 3Y DFS in the NSABP-B27 trial (5Y DFS, 71%) was comparable to that of the neo-shorter subjects treated with AC4-D4 (74.9%). The difference may be due to the presence of higher-risk patients in our current series, including those with more than 4 LN metastases [≥4LN metastases: Neo-Shorter vs. NSABP-B27, 85/247 (34.4%) vs. 114/752 (15.2%)].
In our current cohort, febrile neutropenia was within the 11–34% range reported in previous studies [9, 28]. A numerically larger number of patients withdrew their consent in AC4-D4 (n = 13 in AC4-D4, n = 3 in FEC3-D3). This withdrawal of consent was not necessarily related to the development of adverse events since there was a similar incidence of adverse effects between the two arms. Patients’ change of mind due to personal reasons unrelated to medical reasons was observed in four cases in AC4-D4 and two cases in FEC3-D3. Four patients in AC4-D4 requested to discontinue the chemotherapy due to individual intolerance rather than direct adverse effects, while there were none in FEC3-D3. A numerically high number of HER2-positive patients could not get trastuzumab added to docetaxel due to reimbursement issues (n = 5 in AC4-D4, n = 1 in FEC3-D3). Generally, myalgia is mainly known to be related to docetaxel. In this study, interestingly, myalgia was more common in the fewer cycles of docetaxel arm. Myalgia could occur by chance, but we assumed that the relatively lower dose modification in the FEC3-D3 group as compared with the AC4-D4 group (FEC-D3 vs. AC4-D4; 6/114 [5.2%] vs. 26/104 [25%]), which led to a relatively higher dose intensity (RDI) of docetaxel in the FEC3-D3 group compared with the AC4-D4 group (99.2% in the FEC3-D3 group vs. 97.1% in the AC4-D4 group).
In terms of QoL outcomes in our present cohort, FEC3-D3 was non inferior to AC4-D4 when FACT B scores at all points of time were compared. The FACT-B score, including all subfactors, showed a gradual decrease during chemotherapy. These differences indicated that the chemotherapy affected the QoL. At the treatment baseline, the EWB had the lowest score whereas the FWB score was the lowest at the completion of the chemotherapy. The lowest sub-factor before the start of the chemotherapy was the EWB, and this was likely related to the previously described prevalence of depression in breast cancer patients [32]. That prior study reported that upon a diagnosis of breast cancer, uncertainty about future disease progress, imagining of a poor situation by the patient, and fear of physical changes following treatment can cause depression. In our current investigation, it appeared that the EWB level before the start of chemotherapy was also influenced by the aforementioned factors. The lowest subfactor at the completion of chemotherapy was the FWB, likely because of the toxicity effects after these treatments. Interestingly, at the midpoint of treatment, the FEC3-D3 cases had the lowest SWB and AC4-D4 patients had the lowest FWB. The FWB was thus not the lowest in the FEC3-D3 group even in the middle of the chemotherapy. The cause of this might be associated with the decrease in anthracycline administration but further research is warranted.
Limitations
There were some limitations of our current study of note. Although the results of the TRYPHAENA trial were published in 2013 [33], we were unable to use a HER2 blockade in our current neoadjuvant setting and study period because of the reimbursement policy of the Korean National Health Insurance system for locally advanced breast cancer. Hence, 12.6% (31/247) of the cases included in our present study series had the HER2 subtype. In addition, only the 3Y DFS outcomes could be confirmed among our study patients because of the relatively short follow-up period. In this regard, continuous follow-up will be required to confirm any differences in the long-term outcomes in both arms. Since the sample size was smaller than planned, we could not discriminate whether factors were insignificant due to this reduced sample size or were truly not meaningful. It may thus be necessary to conduct further research in larger cohorts. In this present study, there were fewer patients aged 65 or older (3.2%, 8/247). Considering that aging is a trend in Asian countries, it would be good to have additional studies to confirm our present findings in patients aged 65 or older.
Conclusion
Six cycles of chemotherapy is a potentially viable option for patients who cannot tolerate 8 cycles due to age, time or co-morbidities.
Data availability
All data and materials will be made available upon reasonable request.
References
Fisher B, Brown A, Mamounas E et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National surgical adjuvant breast and bowel project B-18. J Clin Oncol 15(7):2483–2493. https://doi.org/10.1200/jco.1997.15.7.2483
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of National surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26(5):778–785. https://doi.org/10.1200/jco.2007.15.0235
van Nes JG, Putter H, Julien JP, Tubiana-Hulin M, van de Vijver M, Bogaerts J, de Vos M, van de Velde CJ (2009) Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat 115(1):101–113. https://doi.org/10.1007/s10549-008-0050-1
von Minckwitz G, Raab G, Caputo A et al (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German breast group. J Clin Oncol 23(12):2676–2685. https://doi.org/10.1200/jco.2005.05.078
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16(8):2672–2685. https://doi.org/10.1200/jco.1998.16.8.2672
Jones RL, Smith IE (2006) Neoadjuvant treatment for early-stage breast cancer: opportunities to assess tumour response. Lancet Oncol 7(10):869–874. https://doi.org/10.1016/s1470-2045(06)70906-8
Kaufmann M, Hortobagyi GN, Goldhirsch A et al (2006) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol 24(12):1940–1949. https://doi.org/10.1200/jco.2005.02.6187
Bear HD, Anderson S, Brown A et al (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol 21(22):4165–4174. https://doi.org/10.1200/jco.2003.12.005
Roché H, Fumoleau P, Spielmann M et al (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol 24(36):5664–5671. https://doi.org/10.1200/jco.2006.07.3916
Saloustros E, Mavroudis D, Georgoulias V (2008) Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother 9(15):2603–2616. https://doi.org/10.1517/14656566.9.15.2603
Harvey V, Mouridsen H, Semiglazov V, Jakobsen E, Voznyi E, Robinson BA, Groult V, Murawsky M, Cold S (2006) Phase III trial comparing three doses of docetaxel for second-line treatment of advanced breast cancer. J Clin Oncol 24(31):4963–4970. https://doi.org/10.1200/jco.2005.05.0294
Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J (2015) A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol 93(3):203–210. https://doi.org/10.1016/j.critrevonc.2014.10.006
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15(3):974–986. https://doi.org/10.1200/jco.1997.15.3.974
Toi M, Nakamura S, Kuroi K et al (2008) Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat 110(3):531–539. https://doi.org/10.1007/s10549-007-9744-z
Carlson RW, Allred DC, Anderson BO et al (2012) Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 10(7):821–829. https://doi.org/10.6004/jnccn.2012.0086
Heller W, Mazhar D, Ward R et al (2007) Neoadjuvant 5-fluorouracil, epirubicin and cyclophosphamide chemotherapy followed by docetaxel in refractory patients with locally advanced breast cancer. Oncol Rep 17(1):253–259. https://doi.org/10.3892/or.17.1.253
Ohnoa S, Toi M, Kuroi K et al (2005) Update results of FEC followed by docetaxel neoadjuvant trials for primary breast cancer. Biomed Pharmacother 59(Suppl 2):S323-324. https://doi.org/10.1016/s0753-3322(05)80063-0
Chia S, Lohrisch C, Gelmon K et al (2009) Phase II trial of neoadjuvant sequential FEC100 followed by docetaxel and capecitabine for HER2-negative locally advanced breast cancer (LABC): a multicenter study from British Columbia. J Clin Oncol 27:598–598. https://doi.org/10.1200/jco.2009.27.15_suppl.598
Samuel JA, Wilson JW, Bandos H et al (2015) Abstract S3–02: NSABP B-36: a randomized phase III trial comparing six cycles of 5-fluorouracil (5-FU), epirubicin, and cyclophosphamide (FEC) to four cycles of adriamycin and cyclophosphamide (AC) in patients (pts) with node-negative breast cancer. Cancer Res 75(9_Supplement):S3-02-S2-02. https://doi.org/10.1158/1538-7445.sabcs14-s3-02
Del Mastro L, Poggio F, Blondeaux E et al (2022) Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): end-of-study results from a randomised, phase 3 trial. Lancet Oncol 23(12):1571–1582. https://doi.org/10.1016/s1470-2045(22)00632-5
Untch M, Möbus V, Kuhn W et al (2009) Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol 27(18):2938–2945. https://doi.org/10.1200/jco.2008.20.3133
Untch M, von Minckwitz G, Konecny GE et al (2011) PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer–outcome on prognosis. Ann Oncol 22(9):1999–2006. https://doi.org/10.1093/annonc/mdq713
Citron ML, Berry DA, Cirrincione C et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21(8):1431–1439. https://doi.org/10.1200/jco.2003.09.081
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/s0140-6736(13)62422-8
von Minckwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804. https://doi.org/10.1200/jco.2011.38.8595
Asaoka M, Narui K, Suganuma N et al (2019) Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. Eur J Surg Oncol 45(12):2289–2294. https://doi.org/10.1016/j.ejso.2019.08.001
Yee D, DeMichele AM, Yau C et al (2020) Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: 3 year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol 6(9):1355–1362. https://doi.org/10.1001/jamaoncol.2020.2535
Dhanraj KM, Dubashi B, Gollapalli S, Kayal S, Cyriac SL (2015) Comparison of efficacy of neoadjuvant chemotherapy FEC 100 and Docetaxel 75 versus AC and Docetaxel in locally advanced breast cancer: a randomized clinical study. Med Oncol 32(12):261. https://doi.org/10.1007/s12032-015-0697-5
Denkert C, Loibl S, Müller BM et al (2013) Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol 24(11):2786–2793. https://doi.org/10.1093/annonc/mdt350
Yao Y, Chu Y, Xu B, Hu Q, Song Q (2019) Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci Rep. https://doi.org/10.1042/bsr20190288
Salvo EM, Ramirez AO, Cueto J, Law EH, Situ A, Cameron C, Samjoo IA (2021) Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: a systematic review and meta-analysis. Breast 57:5–17. https://doi.org/10.1016/j.breast.2021.02.009
Purkayastha D, Venkateswaran C, Nayar K, Unnikrishnan UG (2017) Prevalence of depression in breast cancer patients and its association with their quality of life: a cross-sectional observational study. Indian J Palliat Care 23(3):268–273. https://doi.org/10.4103/ijpc.ijpc_6_17
Schneeweiss A, Chia S, Hickish T et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24(9):2278–2284. https://doi.org/10.1093/annonc/mdt182
Acknowledgements
This study was presented in part during the 2020 San Antonio Breast Cancer Symposium (SABCS), 8-11 December 2020, San Antonio, Texas, USA.
Funding
This work was supported by a grant from the Sanofi-Aventis (Grant No.2012–0116).
Author information
Authors and Affiliations
Contributions
All of the listed study authors contributed to this study in accordance with the International Committee of Medical Journal Editors (ICMJE) guidelines for authorship. All authors have read and approved the submitted version of the manuscript (and any substantially modified version that involves their contribution to the study). Each author has agreed to be personally accountable for their own contributions and to ensuring that any questions regarding the accuracy or integrity of any part of the work, even those areas in which the author was not personally involved, are appropriately investigated and resolved, and that this resolution is documented in the literature.
Corresponding author
Ethics declarations
Conflict of interest
HJL is founder of Neogene TC. KHJ has advisory roles at Astra-Zeneca, BIXINK, MSD, Novartis, Pfizer, Roche, Takeda and Everest Medicine. SBK is a consultant on the advisory boards of Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi-Sankyo, and has received research funding from Novartis, Sanofi-Aventis, and DongKook Pharm Co., and owns stock in Genopeaks and NeogeneTC. No other authors have any conflicts of interest to declare in relation to this study.
Ethical approval and consent to participate
The Institutional Review Board (IRB) at our institution approved this study. All procedures involving human participants followed the ethical standards of the institutional and/or national research committee, and of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving human and animal participants
All of the enrolled human subjects in this study provided written informed consent to participate and to the publication of the findings.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors confirm that this manuscript does not contains any identifying personal information regarding the study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hwang, I., Kim, J.E., Jeong, J.H. et al. Randomized phase III trial of a neoadjuvant regimen of four cycles of adriamycin plus cyclophosphamide followed by four cycles of docetaxel (AC4-D4) versus a shorter treatment of three cycles of FEC followed by three cycles of docetaxel (FEC3-D3) in node-positive breast cancer (Neo-shorter; NCT02001506). Breast Cancer Res Treat 201, 193–204 (2023). https://doi.org/10.1007/s10549-023-06971-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06971-7