Abstract
Purpose
This study aimed to examine the clinical characteristics and outcomes of patients with estrogen receptor-negative (ER−)/progesterone receptor-positive (PR+) early breast cancer. We also aimed to investigate the benefits of adjuvant endocrine therapy (ET) in this patient population.
Methods
Patients with early breast cancer diagnosed at West China Hospital were divided into the ER−/PR+, ER+, and ER−/PR− groups. The chi-square test was used to analyze differences in clinical and pathological features among the groups. Multivariable Cox and Fine–Gray regression models were used to compare mortality and locoregional recurrence (LRR)/distant recurrence (DR), respectively. We performed a subgroup analysis to determine which ER−/PR+ patients can benefit more from ET.
Results
From 2008 to 2020, we enrolled 443, 7104, and 2892 patients into the ER−/PR+, ER+, and ER−/PR− groups, respectively. The ER−/PR+ group showed more unfavorable clinical features and aggressive pathological characteristics than the ER+ group. The mortality, LRR, and DR rates were higher in the ER−/PR+ than in the ER+ group. Most clinical features and pathological characteristics were similar between the ER−/PR+ and ER−/PR− group and their outcomes were comparable. In the ER−/PR+ group, patients who received ET showed significantly lower LRR and mortality rates than those who did not; however, no difference was observed in DR. Subgroup analysis suggested that ER−/PR+ patients age ≥ 55 years, and postmenopausal status can benefit from ET.
Conclusion
ER−/PR+ tumors have more aggressive pathological characteristics and more unfavorable clinical features than ER+ tumors. ET can reduce the LRR and mortality rates in ER−/PR+ patients. Postmenopausal and age ≥ 55 years ER−/PR+ patients can benefit from ET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For patients with early-stage breast cancer (BC) with positive hormone receptor status, 5–10 years of adjuvant endocrine therapy (ET) can significantly reduce the recurrence and mortality rates. Immunohistochemistry (IHC) testing for hormone receptor status is recommended for patients with newly diagnosed primary or metastatic BC [1].
Patients with estrogen receptor (ER)-positive BC (ER expression 1–100%) are known to benefit from ET. Patients with ER-negative/progesterone receptor (PR)-positive BC may be considered for ET; however, limited data are available for this patient group because ER−/PR+ BC accounts for < 10% of all BC cases [2,3,4]. Owing to the rarity of this subtype, few studies have assessed the response to ET in ER−/PR+ patients, and many prospective studies excluded this patient population [5].
Some studies have suggested that the ER−/PR+ subtype is biologically implausible given the co-expression pathway of ER and PR in BC [6, 7]. Other studies have indicated that most ER−/PR+ BC cases may represent false-negative IHC results for ER [8]. However, some studies have also reported that the mechanism of positive PR expression in ER- cases may be explained by the predominance of a variant form of ER [9, 10], the presence of ER missense mutations [11], or the activation of an alternative pathway [12]. Additionally, a study of ER−/PR+ BC cell lines demonstrated that PR can be expressed independently of the regulatory mechanisms of ER [13]. Thus, ER−/PR+ BC may represent a rare biological entity [14].
Whether ER−/PR+ patients can benefit from ET is highly controversial. A previous study indicated that patients with ER−/PR+ BC could benefit from tamoxifen therapy [15]. Another study concluded that adjuvant tamoxifen therapy might not provide a survival benefit for patients with high-grade ER−/PR+ tumors but was recommended for patients with low-grade ER−/PR+ tumors [16]. Conversely, a meta-analysis showed that only ER status, not PR status, was statistically significantly associated with tamoxifen response [5]. Another study reported that patients with ER−/PR+ BC who received ET had shorter survival times than those who did not [17].
In this study, we investigated a prospective cohort of 10,439 patients with early-stage BC diagnosed at West China Hospital (WCH) between 2008 and 2020. We compared the clinical-pathological features and survival outcomes of ER−/PR+ patients with those of ER+ and ER−/PR− patients. We also investigate the benefits of ET in the ER−/PR+ patient population.
Methods
Study design
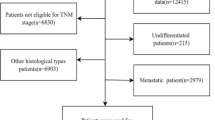
A flowchart of the study design and patient selection process is shown in Fig. 1.
Study population
Since 2008, patients with BC have been prospectively enrolled in the Breast Cancer Information Management System (BCIMS) of the WCH of Sichuan University [18]. Physicians collected medical records, pathological diagnosis information, and treatment data. Outpatient or telephone follow-up was performed every 3–4 months for the first 2 years, every 6 months for the next 3–5 years, and every year thereafter. This study was approved by the Clinical Test and Biomedical Ethics Committee of WCH, Sichuan University (reference no. 2012-130). All patients provided written informed consent. From 2008 to 2020, a total of 12,112 patients were registered in the BCIMS. Patients with no ER or PR status information, male sex, no survival information, experienced early events within 3 months or had < 3 months of follow-up, stage IV disease or no stage information, no or palliative surgery, or bilateral BC were excluded. Finally, 10,439 patients were included in this study.
Data on demographic features (age, residence, educational level, menopausal status, and body mass index [BMI]), clinical characteristics (human epidermal growth factor receptor 2 [HER2] status, Ki67 expression, CK5/6 status, tumor-node-metastasis [TNM] stage, histological type, and grade), and treatment modes (ET, chemotherapy, and radiotherapy) were collected.
Pathological diagnosis and IHC
All pathological evaluations and IHC tests were performed at our hospital. Using antibodies selected by our institution, IHC was performed by staining for ER, PR, and HER2 on paraffin-embedded slides after deparaffinization, rehydration, and antigen retrieval [19]. ER or PR status was defined according to the percentage of tumor cells that positively expressed ER or PR; tumors with ≥ 1% stained cells nuclei were considered positive. The staining intensity of ER or PR was not included in our study. HER2 status was initially assessed using IHC and scored using a semi-quantitative scoring system. The status was confirmed using fluorescence in situ hybridization (FISH) in IHC equivocal cases (score 2+) according to the 2007 American Society of Clinical Oncology/College of American Pathologists guidelines [20]. HER2 IHC 0–1+ or FISH-negative tumors were considered negative; IHC 3+ or FISH-positive tumors were considered positive; and IHC 2+ tumors without FISH results were considered to have an uncertain status.
ESR1 mRNA expression
ESR1 mRNA expression was detected using next-generation sequencing in 14 ER−/PR+ and 128 ER+ patients. RNA sequencing of frozen tumor specimens was performed on the Illumina Novaseq S6000 platform, as previously described. After quality control, the readings were mapped to the reference genome using HISAT2 version 2.0.5 [21]. The fragments per kilobase of exon per million mapped fragments (FPKM) values of ESR1, representing ER mRNA expression, were calculated according to a previously described method [22]. This part of the study was separately approved by the Clinical Test and Biomedical Ethics Committee of WCH, Sichuan University (reference no. 2019-16).
Outcome assessment and statistical analysis
Mortality, locoregional recurrence (LRR), and distant recurrence (DR) were defined as death from any cause, tumor recurrence in the ipsilateral chest wall or regional lymph nodes, and disease recurrence in distant organs, respectively.
The patients were classified into three groups: ER−/PR+, ER+, and ER−/PR−. The chi-square test was used to compare the demographic features, clinical characteristics, and treatment modes among the three groups. The t-test was used to compare intergroup differences between two continuous variables, and two-way analysis of variance was used to compare intergroup differences among three or more continuous variables. A univariate analysis was performed to determine which covariates to adjust for in a multivariable analysis using Cox proportional hazard or Fine–Gray competing risk regression models. Mortality incidence curves were constructed using Kaplan–Meier survival analysis, and differences between groups were compared using the log-rank test. The Fine–Gray competing risk regression was used to compare the LRR and DR rates between groups. Death from any cause was considered a competing risk event for LRR and DR. R version 4.1.0 was used for statistical analysis. A two-tailed P value of < 0.05 was considered statistically significant.
Results
Proportion of ER−/PR+ patients
Among the 10,439 patients included in this study, 443 (4.25%) were ER−/PR+, 7104 (68.05%) were ER+, and 2892 (27.7%) were ER−/PR−. The last follow-up date was November 2021. The IHC stain of ER and PR in the ER−/PR+ group was shown in supplemental Fig. 1. We reviewed the proportion of ER−/PR+ patients every year during the study period. The proportion of ER−/PR+ patients varied from 1.41% in 2009 to 9.68% in 2014 (Supplemental Fig. 2a). We also compared the ESR1 mRNA expression levels between ER−/PR+ and ER+ patients. We observed that the ESR1 mRNA expression levels in 14 ER−/PR+ patients were significantly lower than those in 128 ER+ patients (P = 1.3E−08) (Supplemental Fig. 2b), suggesting that the ER−/PR+ status cannot be explained by false-negative staining for ER. In addition, the percentage of PR-expressing cells was significantly lower in ER−/PR+ tumors than in ER+/PR+ tumors. In 82.84% of ER−/PR+ tumors, 1–20% of cells expressed PR, indicating that most of the tumor cells may be ER−/PR−. Moreover, the tumors in this group had a high degree of intra-tissue heterogeneity (Supplemental Fig. 2c).
Demographic features, clinicopathological characteristics, and treatment modes
Table 1 shows that ER−/PR+ patients were more frequently aged ≥ 55 years (31.2% vs. 24.9%) and more likely to be postmenopausal (51.5% vs. 41.1%) than ER+ patients. The ER−/PR+ group also had a higher TNM stage (stage III, 34.8% vs. 27.5%), a higher proportion of HER2-positive patients (43.8% vs. 19.9%), higher Ki67 expression (Ki67 ≥ 30%) (77.8% vs. 50.7%), and a higher proportion of CK5/6-positive patients (45.5% vs. 5.8%) than the ER+ group. More importantly, the ER−/PR+ group had more patients with grade 3 tumors (78.6% vs. 44.8%) than the ER+ group. Fewer patients received ET (63.2% vs. 91%), but more patients received chemotherapy (96.6% vs. 92.8%) in the ER−/PR+ group than in the ER+ group.
ER−/PR− and ER−/PR+ patients showed similar age, educational characteristics, menopausal status, HER2 status, Ki67% expression, and CK5/6 status; however, ER−/PR+ patients were more likely to have stage III disease (34.8% vs. 29%). Moreover, the proportion of patients with grade 3 tumors was smaller in the ER−/PR+ group than in the ER−/PR− group (78.6% vs. 83.6%). In terms of treatment mode, more ER−/PR+ patients than ER−/PR− patients received ET (63.2% vs. 7.3%) (Table 1). These results indicated that ER−/PR+ tumors had more aggressive and unfavorable characteristics than ER+ tumors but showed similar characteristics to ER−/PR− tumors.
Survival analysis of ER−/PR+, ER+, and ER−/PR− patients
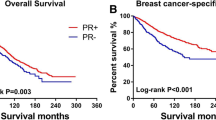
The median follow-up time for the prospective cohort was 65.3 months. Of the patients, 472 died from any cause, 197 had LRR, 937 had DR, and 117 had both LRR and DR. The 5-year mortality rates were 9.0%, 3.1%, and 7.5% in the ER−/PR+, ER+, and ER−/PR− groups, respectively (log-rank P < 0.001). The 5-year LRR rates were 5.6%, 1.3%, and 2.9% in the ER−/PR+, ER+, and ER−/PR− groups, respectively (Gray’s test P < 0.001). The 5-year DR rates were 16.0%, 7.7%, and 13% in the ER−/PR+, ER+, and ER−/PR− groups, respectively (Gray’s test P < 0.001) (Table 2). ER+ patients presented a lower mortality risk (hazard ratio [HR] 0.41, 95% confidence interval [CI] 0.28–0.61, P = 7.5E−06), LRR risk (HR 0.26, 95% CI 0.15–0.44, P = 6.6E−07), and DR risk (HR 0.58, 95% CI 0.42–0.81, P = 0.001) than ER−/PR+ patients. However, no significant differences in mortality (HR 0.82, 95% CI 0.56–1.2, P = 0.3) and DR (HR 0.88, 95% CI 0.63–1.24, P = 0.77) were observed between the ER−/PR− and ER−/PR+ groups. Surprisingly, the LRR risk was significantly lower (HR 0.43, 95% CI 0.23–0.8, P = 0.007) in the ER−/PR− group than in the ER−/PR+ group. In multivariable Cox regression and Fine–Gray competing risk regression analyses, HRs and P values were adjusted for covariates that had a P value of < 0.1 in the univariate analysis. The tumor grade was not adjusted because tumor grade information was not available for a considerable number of patients. (Fig. 2a–c, Table 3, and Supplemental Tables 1, 2, 3).
Survival analysis of ER−/PR+, ER+, and ER−/PR− patients treated with ET
After ET, the ER+ group still showed better outcomes in terms of mortality, LRR, and DR than the ER−/PR+ group. However, no significant differences in mortality, LRR, and DR rates were observed between the ER−/PR+ and ER−/PR− groups. The 5-year mortality rates were 6.1%, 2.7%, and 6.4% in the ER−/PR+, ER+, and ER−/PR− groups, respectively (log-rank P = 0.001). The 5-year LRR rates were 3.6%, 1.2%, and 5.5% in the ER−/PR+, ER+, and ER−/PR− groups, respectively (Gray’s test P < 0.001). The 5-year DR rates were 16%, 7.5%, and 16% in the ER−/PR+, ER+, and ER−/PR− groups, respectively (Gray’s test P < 0.001) (Table 2). The ER+ group still presented a lower risk of mortality (HR 0.51, 95% CI 0.29–0.91, P = 0.022), LRR (HR 0.37, 95% CI 0.19–0.74, P = 0.005), and DR (HR 0.65, 95% CI 0.43–0.97, P = 0.037) than the ER−/PR+ group after ET. However, no differences in mortality (HR 1.2, 95% CI 0.57–2.5, P = 0.64), LRR (HR 1.2, 95% CI 0.51–2.86, P = 0.67), and DR (HR 1.29, 95% CI 0.77–2.14, P = 0.33) were observed between the ER−/PR+ and ER−/PR− groups after ET. In multivariable Cox regression and Fine–Gray competing risk regression analyses, HRs and P values were adjusted for covariates that had a P value of < 0.1 in the univariate analysis, except for tumor grade as mentioned above (Fig. 3a–c, Table 3, Supplemental Tables 4, 5, 6).
Survival analysis of ER−/PR+ patients treated or not with ET and subgroup analysis
ET decreased the mortality rate but not the DR rate in the ER−/PR+ group. A decreased LRR risk was also observed in patients who received ET, with borderline significance by Gray’s test. The 5-year mortality rates were 14.8% and 6.1% in the no ET and ET groups, respectively (log-rank P = 0.004). The 5-year LRR rates were 9.5% and 3.6% in the no ET and ET groups, respectively (Gray’s test P = 0.056). The 5-year DR rates were 15% and 16% in the no ET and ET groups, respectively (Gray’s test P = 0.9) (Table 2). In ER−/PR+ patients, ET reduced the mortality risk by 50% (HR 0.5, 95% CI 0.25–0.98, P = 0.045) and the LRR risk by 60% (HR 0.4, 95% CI 0.18–0.87, P = 0.02) compared with the absence of ET. However, no difference was observed in DR (HR 1.21, 95% CI 0.71–2.05, P = 0.49). In the multivariable Cox regression and Fine–Gray competing risk regression analyses, HRs and P values were adjusted for covariates that had a P value of < 0.1 in the univariate analysis (Fig. 4a–c, Table 3, Supplemental Tables 7, 8, 9).
In addition, we performed a subgroup analysis to determine which subgroup of ER−/PR+ patients can gain a survival benefit from ET. Age, residence, education, menopausal status, and TNM stage had a P value of < 0.1 in the univariate analysis and were used as adjustment variables for the multivariable Cox regression analysis. Patients aged ≥ 55 years and postmenopausal patients gained a survival benefit from ET. Patients aged < 55 years and premenopausal patients did not appear to benefit from ET (for interaction, P values were 0.046 and 0.031, respectively) (Fig. 4d).
Discussion
This prospective cohort study with a large sample size describes the survival rate of patients with ER−/PR+ early BC in WCH from 2008 to 2020. We found that the ER−/PR+ subtype was associated with more unfavorable clinical features and more aggressive pathological characteristics compared with ER+ BC but showed similar characteristics to ER−/PR− BC. The mortality, LRR, and DR rates of ER−/PR+ patients were significantly worse than those of ER+ patients, even after ET. Nevertheless, ET reduced the mortality and LRR risks, but not the DR risks, in ER−/PR+ patients. Furthermore, the mortality risk was significantly reduced by ET in patients with age ≥ 55 years, and postmenopausal status.
The proportion of ER−/PR+ patients in our database (4.24%) was higher than that in the Surveillance, Epidemiology, and End Results database (SEER) (1.6%) [23]. The difference between the two databases may be due to race because a retrospective study from South Korea showed that ER−/PR+ patients accounted for 9.4% of all patients with BC [24] and another study from China reported that the proportion of ER−/PR+ patients was 11% [25]. The proportion of PR−/ER+ patients in our study did not decrease with time, thereby excluding the possibility that the difference in proportion was caused by advances in IHC technology. In addition, the RNA sequencing data showed that the ESR1 FPKM value was significantly lower in ER−/PR+ patients than in ER+ patients, suggesting that the classification of ER−/PR+ patients was not caused by false-negative staining for ER. We also observed that the PR expression percentage was significantly higher in ER+ patients than in ER−/PR+ patients, and > 80% of ER−/PR+ patients had PR expression between 1 and 20%. This indicates that other alternative mechanisms may be responsible for the expression of PR in ER−/PR+ patients. We noted that in 2013 WCH changed the ER and PR antibodies for pathological diagnosis, which may weaken the robustness of our results.
Similar to other studies, our results demonstrated that ER−/PR+ BC had more unfavorable clinical features and more aggressive biological characteristics, such as a higher stage, a higher tumor grade, a higher proportion of CK5/6-positive status [4], higher HER2 expression [26], and higher Ki67 expression [24, 27], than ER+ BC. Consistent with most studies [28, 29], our results also indicated that ER−/PR+ patients had a higher risk of mortality, LRR, and DR than ER+ patients, even after ET [25].
However, whether a survival difference exists between the ER−/PR+ and ER−/PR− groups remains controversial. Most studies indicated that ER−/PR+ patients have a better prognosis than ER−/PR− patients [16, 23, 30]. Other studies demonstrated no difference in survival between the ER−/PR+ and ER−/PR− groups [29]. Meanwhile, some studies demonstrated that ER−/PR+ patients have lower disease-specific survival rates than ER−/PR− patients [31]. We observed similar mortality and DR rates between the ER−/PR+ group and in the ER−/PR− group, although a higher LRR rate was observed in the ER−/PR+ patients. Several possible reasons can be suggested to explain these results. First, the expression percentage of PR was 1–20% in > 80% of ER−/PR+ patients, indicating that the tumor tissues predominantly consisted of ER−/PR− cells. Therefore, the prognosis may be similar between the ER−/PR+ and ER−/PR− groups. Second, in our cohort, ER−/PR+ patients had a higher TNM stage than ER−/PR− patients. There were more stage III patients and fewer stage 0–I patients in the ER−/PR+ group than in ER−/PR− group. Third, one-third of ER−/PR+ patients did not receive ET, which might have improved their survival rate.
Although the predictive value of ER in patients with early BC treated with ET is widely recognized, the predictive and prognostic significance of PR is still a topic of debate [32, 33]. Controversy remains about whether ET can improve the prognosis of ER−/PR+ patients. Dowsett et al. reported that PR+ patients could significantly benefit from tamoxifen treatment [34], and another study demonstrated that ET could improve relapse-free and overall survival [35]. However, a meta-analysis showed no benefit from tamoxifen therapy in patients with ER-poor BC, irrespective of the PR status [5]. Yang et al. suggested that in the ER−/PR+ group, only patients with low-grade tumors showed better overall and disease-free survival after ET [16]. Another study reported that ER−/PR+ patients who received ET had shorter survival times than those who did not [17].
In our cohort, ER−/PR+ patients who received ET showed significantly decreased mortality and LRR rates compared with those who did not receive ET; however, the DR rates did not differ between the two groups. A previous study reported that PR loss is more common than ER and HER2 loss in recurrent metastatic disease [36]. In other words, metastatic cell colonies may form mainly from ER−/PR− cells, which may explain why ET can significantly reduce local recurrence but not distant metastasis. A small number of ER−/PR− BC patients (7.3%) in our study cohort received ET. This may be due to inconsistent results between the core needle biopsy and the postoperative specimen or a second hormone receptor-positive BC.
Yamashita et al. reported that patients with an ER or PR expression percentage of ≥ 1% had better survival after relapse and suggested 1% as the cutoff value [37]. However, our subgroup analysis demonstrated that when the PR expression percentage was ≥ 10%, ET significantly reduced the risk of mortality in the ER−/PR+ group. The P for interaction was > 0.05, which may be due to the small number of ER−/PR+ patients. Therefore, this result may need to be confirmed in a study with a larger sample size. More randomized controlled studies are needed to decide the optimal PR threshold for making ET decisions. Because of the limited number of ER−/PR+ cases and some of patients’ HER2 status were equivocal, we did not stratify patients by HER2 status for survival analysis. Subgroup analysis suggested that HER2 status did not appear to alter the results of patients who used ET or not.
In addition, postmenopausal patients and those aged ≥ 55 years seemed to have significantly benefited from ET in terms of mortality compared with patients with a premenopausal status and age < 55 years. Our results provide a good basis for selecting ER−/PR+ patients for ET treatment.
Conclusion
Our results demonstrated that ER−/PR+ tumors had more unfavorable clinical features and aggressive pathological characteristics than ER+ tumors. The prognosis in terms of mortality, LRR, and DR was worse in the ER−/PR+ group than in the ER+ group, even after ET. However, most clinical features and pathological characteristics were similar between the ER−/PR+ and ER−/PR− group and their outcomes were comparable. The LRR and mortality rates were reduced by ET in ER−/PR+ patients. Subgroup analysis suggested that patients age ≥ 55 years, and postmenopausal status can significantly benefit from ET.
Data availability
Data supporting the findings of this study are available upon request from the corresponding author.
Abbreviations
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- ET:
-
Adjuvant endocrine therapy
- LRR:
-
Locoregional recurrence
- DR:
-
Distant recurrence
- BC:
-
Breast cancer
- IHC:
-
Immunohistochemistry
- BCIMS:
-
Breast Cancer Information Management System
- WCH:
-
West China Hospital
- BMI:
-
Body mass index
- HER2:
-
Human epidermal growth factor receptor 2
- TNM:
-
Tumor-node-metastasis
- FISH:
-
Fluorescence in situ hybridization
- FPKM:
-
Fragments per kilobase of exon per million mapped fragments
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 38:1346–1366. https://doi.org/10.1200/JCO.19.02309
Rhodes A, Jasani B, Balaton AJ, Barnes DM, Miller KD (2000) Frequency of oestrogen and progesterone receptor positivity by immunohistochemical analysis in 7016 breast carcinomas: correlation with patient age, assay sensitivity, threshold value, and mammographic screening. J Clin Pathol 53:688–696. https://doi.org/10.1136/jcp.53.9.688
Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS, Hershman D, Neugut AI (2005) Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer 103:2241–2251. https://doi.org/10.1002/cncr.21030
Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, Nicholson RI, Lee AH, Robertson JF, Ellis IO (2007) Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol 25:4772–4778. https://doi.org/10.1200/JCO.2007.12.2747
Early Breast Cancer Trialists’ Collaborative G, Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
Horwitz KB, McGuire WL (1978) Estrogen control of progesterone receptor in human breast cancer. correlation with nuclear processing of estrogen receptor. J Biol Chem 253:2223–2228
Onitilo AA, Engel J, Joseph AO, Li YH (2021) Is oestrogen receptor-negative/progesterone receptor-positive (ER−/PR+) a real pathological entity? Ecancermedicalscience 15:1278. https://doi.org/10.3332/ecancer.2021.1278
Cserni G, Francz M, Kalman E, Kelemen G, Komjathy DC, Kovacs I, Kulka J, Sarkadi L, Udvarhelyi N, Vass L, Voros A (2011) Estrogen receptor negative and progesterone receptor positive breast carcinomas-how frequent are they? Pathol Oncol Res 17:663–668. https://doi.org/10.1007/s12253-011-9366-y
Fuqua SA, Fitzgerald SD, Chamness GC, Tandon AK, McDonnell DP, Nawaz Z, O’Malley BW, McGuire WL (1991) Variant human breast tumour estrogen receptor with constitutive transcriptional activity. Cancer Res 51:105–109
Al-Bader M, Al-Saji S, Ford CH, Francis I, Al-Ayadhy B (2010) Real-time PCR: detection of oestrogen receptor-alpha and -beta isoforms and variants in breast cancer. Anticancer Res 30:4147–4156
Herynk MH, Fuqua SA (2004) Estrogen receptor mutations in human disease. Endocr Rev 25:869–898. https://doi.org/10.1210/er.2003-0010
Kunc M, Biernat W, Senkus-Konefka E (2018) Estrogen receptor-negative progesterone receptor-positive breast cancer—“Nobody’s land” or just an artifact? Cancer Treat Rev 67:78–87. https://doi.org/10.1016/j.ctrv.2018.05.005
Borras M, Lacroix M, Legros N, Leclercq G (1997) Estrogen receptor-negative/progesterone receptor-positive Evsa-T mammary tumour cells: a model for assessing the biological property of this peculiar phenotype of breast cancers. Cancer Lett 120:23–30. https://doi.org/10.1016/s0304-3835(97)00285-1
Ng CH, Pathy NB, Taib NA, Mun KS, Rhodes A, Yip CH (2012) The estrogen receptor negative-progesterone receptor positive breast carcinoma is a biological entity and not a technical artifact. Asian Pac J Cancer Prev 13:1111–1113. https://doi.org/10.7314/apjcp.2012.13.4.1111
Colomer R, Beltran M, Dorcas J, Cortes-Funes H, Hornedo J, Valentin V, Vargas C, Mendiola C, Ciruelos E (2005) It is not time to stop progesterone receptor testing in breast cancer. J Clin Oncol 23:3868–3869. https://doi.org/10.1200/JCO.2005.05.203. (author reply 3869–3870)
Yang LH, Tseng HS, Lin C, Chen LS, Chen ST, Kuo SJ, Chen DR (2012) Survival benefit of tamoxifen in estrogen receptor-negative and progesterone receptor-positive low grade breast cancer patients. J Breast Cancer 15:288–295. https://doi.org/10.4048/jbc.2012.15.3.288
Schroth W, Winter S, Buttner F, Goletz S, Faisst S, Brinkmann F, Saladores P, Heidemann E, Ott G, Gerteis A, Alscher MD, Dippon J, Schwab M, Brauch H, Fritz P (2016) Clinical outcome and global gene expression data support the existence of the estrogen receptor-negative/progesterone receptor-positive invasive breast cancer phenotype. Breast Cancer Res Treat 155:85–97. https://doi.org/10.1007/s10549-015-3651-5
Fan Y, Xie G, Wang Z, Wang Y, Wang Y, Zheng H, Zhong X (2022) PTEN promoter methylation predicts 10-year prognosis in hormone receptor-positive early breast cancer patients who received adjuvant tamoxifen endocrine therapy. Breast Cancer Res Treat 192:33–42. https://doi.org/10.1007/s10549-021-06463-6
Luo C, Zhong X, Fan Y, Wu Y, Zheng H, Luo T (2022) Clinical characteristics and survival outcome of patients with estrogen receptor low positive breast cancer. Breast 63:24–28. https://doi.org/10.1016/j.breast.2022.03.002
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical Oncology/College of American P (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 131:18–43. https://doi.org/10.5858/2007-131-18-ASOCCO
Hu K, Wang C, Luo C, Zheng H, Song H, Bergstedt J, Fall K, Luo T, Czene K, Valdimarsdottir UA, Fang F, Lu D (2022) Neuroendocrine pathways and breast cancer progression: a pooled analysis of somatic mutations and gene expression from two large breast cancer cohorts. BMC Cancer 22:680. https://doi.org/10.1186/s12885-022-09779-8
Zhao Y, Li MC, Konate MM, Chen L, Das B, Karlovich C, Williams PM, Evrard YA, Doroshow JH, McShane LM (2021) TPM, FPKM, or normalized counts? A comparative study of quantification measures for the analysis of RNA-seq data from the NCI patient-derived models repository. J Transl Med 19:269. https://doi.org/10.1186/s12967-021-02936-w
Li Y, Yang D, Yin X, Zhang X, Huang J, Wu Y, Wang M, Yi Z, Li H, Li H, Ren G (2020) Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer. JAMA Netw Open 3:e1918160. https://doi.org/10.1001/jamanetworkopen.2019.18160
Park S, Park BW, Kim TH, Jeon CW, Kang HS, Choi JE, Hwang KT, Kim IC (2013) Lack of either estrogen or progesterone receptor expression is associated with poor survival outcome among luminal A breast cancer subtype. Ann Surg Oncol 20:1505–1513. https://doi.org/10.1245/s10434-012-2772-x
Yu KD, Di GH, Wu J, Lu JS, Shen KW, Liu GY, Shen ZZ, Shao ZM (2008) Breast cancer patients with estrogen receptor-negative/progesterone receptor-positive tumours: being younger and getting less benefit from adjuvant tamoxifen treatment. J Cancer Res Clin Oncol 134:1347–1354. https://doi.org/10.1007/s00432-008-0414-2
Chan M, Chang MC, Gonzalez R, Lategan B, del Barco E, Vera-Badillo F, Quesada P, Goldstein R, Cruz I, Ocana A, Cruz JJ, Amir E (2015) Outcomes of estrogen receptor negative and progesterone receptor positive breast cancer. PLoS ONE 10:e0132449. https://doi.org/10.1371/journal.pone.0132449
Itoh M, Iwamoto T, Matsuoka J, Nogami T, Motoki T, Shien T, Taira N, Niikura N, Hayashi N, Ohtani S, Higaki K, Fujiwara T, Doihara H, Symmans WF, Pusztai L (2014) Estrogen receptor (ER) mRNA expression and molecular subtype distribution in ER-negative/progesterone receptor-positive breast cancers. Breast Cancer Res Treat 143:403–409. https://doi.org/10.1007/s10549-013-2763-z
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumour characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9:R6. https://doi.org/10.1186/bcr1639
Yu KD, Jiang YZ, Hao S, Shao ZM (2015) Molecular essence and endocrine responsiveness of estrogen receptor-negative, progesterone receptor-positive, and HER2-negative breast cancer. BMC Med 13:254. https://doi.org/10.1186/s12916-015-0496-z
Liu S, Chia SK, Mehl E, Leung S, Rajput A, Cheang MC, Nielsen TO (2010) Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat 119:53–61. https://doi.org/10.1007/s10549-009-0318-0
Yi M, Mittendorf EA, Cormier JN, Buchholz TA, Bilimoria K, Sahin AA, Hortobagyi GN, Gonzalez-Angulo AM, Luo S, Buzdar AU, Crow JR, Kuerer HM, Hunt KK (2011) Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol 29:4654–4661. https://doi.org/10.1200/JCO.2011.38.3174
MacGrogan G, de Mascarel I, Sierankowski G, Mauriac L, Debled M, Durand M, De Lara CT, Avril A, Picot V, Mathoulin-Pelissier S (2005) Time for reappraisal of progesterone-receptor testing in breast cancer management. J Clin Oncol 23:2870–2871. https://doi.org/10.1200/JCO.2005.05.241. (author reply 2871)
Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell’Orto P, Rasmussen BB, Raffoul J, Neven P, Orosz Z, Braye S, Ohlschlegel C, Thurlimann B, Gelber RD, Castiglione-Gertsch M, Price KN, Goldhirsch A, Gusterson BA, Coates AS (2007) Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol 25:3846–3852. https://doi.org/10.1200/JCO.2007.11.9453
Dowsett M, Houghton J, Iden C, Salter J, Farndon J, A’Hern R, Sainsbury R, Baum M (2006) Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol 17:818–826. https://doi.org/10.1093/annonc/mdl016
Fan Y, Ding X, Xu B, Ma F, Yuan P, Wang J, Zhang P, Li Q, Luo Y (2015) Prognostic significance of single progesterone receptor positivity: a comparison study of estrogen receptor negative/progesterone receptor positive/Her2 negative primary breast cancer with triple negative breast cancer. Medicine 94:e2066. https://doi.org/10.1097/MD.0000000000002066
Rossi S, Basso M, Strippoli A, Dadduzio V, Cerchiaro E, Barile R, D’Argento E, Cassano A, Schinzari G, Barone C (2015) Hormone receptor status and HER2 expression in primary breast cancer compared with synchronous axillary metastases or recurrent metastatic disease. Clin Breast Cancer 15:307–312. https://doi.org/10.1016/j.clbc.2015.03.010
Yamashita H, Yando Y, Nishio M, Zhang Z, Hamaguchi M, Mita K, Kobayashi S, Fujii Y, Iwase H (2006) Immunohistochemical evaluation of hormone receptor status for predicting response to endocrine therapy in metastatic breast cancer. Breast Cancer 13:74–83. https://doi.org/10.2325/jbcs.13.74
Funding
This work was supported by the key program of Science & Technology Department of Sichuan Province (Grant Number: 2017SZ0005, to H.Z.); Key research and development projects of Science & Technology Department of Sichuan Province (Grant Number: 2019YFS0342, to Y.F.).
Author information
Authors and Affiliations
Contributions
YF: Literature review; statistical analysis and interpretation of data; drafting of the manuscript; review of the manuscript for important intellectual content and final approval of the version to be submitted. YW, ZW: Collection, preservation, sorting and delivery of frozen specimens for sequencing and approval of the version to be submitted. XZ and TL: Clinical data collection and collation, revision, and approval of the version to be submitted. HZ and YW: Study concept; review of the manuscript for important intellectual content and final approval of the version to be submitted.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Clinical Test and Biomedical Ethics Committee at the West China Hospital, Sichuan University (Reference Number 2012–130). Next-generation sequencing was approved by the Clinical Test and Biomedical Ethics Committee at the West China Hospital, Sichuan University (Reference Number 2019-16).
Consent to participate
All patients provided written informed consent forms.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fan, Y., Zhong, X., Wang, Y. et al. A prospective cohort study of clinical characteristics and outcomes in Chinese patients with estrogen receptor-negative/progesterone receptor-positive early breast cancer. Breast Cancer Res Treat 200, 171–182 (2023). https://doi.org/10.1007/s10549-023-06964-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06964-6