Abstract
Purpose
Evaluate the COVID-19 pandemic impact on breast cancer detection method, stage and treatment before, during and after health care restrictions.
Methods
In a retrospective tertiary cancer care center cohort, first primary breast cancer (BC) patients, years 2019–2021, were reviewed (n = 1787). Chi-square statistical comparisons of detection method (patient (PtD)/mammography (MamD), Stage (0-IV) and treatment by pre-pandemic time 1: 2019 + Q1 2020; peak-pandemic time 2: Q2-Q4 2020; pandemic time 3: Q1-Q4 2021 (Q = quarter) periods and logistic regression for odds ratios were used.
Results
BC case volume decreased 22% in 2020 (N = 533) (p = .001). MamD declined from 64% pre-pandemic to 58% peak-pandemic, and increased to 71% in 2021 (p < .001). PtD increased from 30 to 36% peak-pandemic and declined to 25% in 2021 (p < .001). Diagnosis of Stage 0/I BC declined peak-pandemic when screening mammography was curtailed due to lock-down mandates but rebounded above pre-pandemic levels in 2021. In adjusted regression, peak-pandemic stage 0/I BC diagnosis decreased 24% (OR = 0.76, 95% CI: 0.60, 0.96, p = .021) and increased 34% in 2021 (OR = 1.34, 95% CI: 1.06, 1.70, p = .014). Peak-pandemic neoadjuvant therapy increased from 33 to 38% (p < .001), primarily for surgical delay cases.
Conclusions
The COVID-19 pandemic restricted health-care access, reduced mammography screening and created surgical delays. During the peak-pandemic time, due to restricted or no access to mammography screening, we observed a decrease in stage 0/I BC by number and proportion. Continued low case numbers represent a need to re-establish screening behavior and staffing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first reported case of 2019-nCoV infection in the United States was in Washington State on January 19, 2020 and on March 23rd 2020, a statewide stay-at-home order was announced to remain in effect until May 30, 2020 and then lifted in a move to phased reopening [1, 2]. On March 19, 2020 statewide non-urgent procedures were prohibited in hospitals and ambulatory surgical facilities until May 18, 2020 [3]. Physician groups recommended postponement of breast cancer screening and some recommended delays set by a priority system [4, 5]. Most health care systems including ours started reopening to screening in the summer of 2020 after stay-at-home orders were lifted in May 2020 [3, 6].
In Washington State and in particular at our institution restrictions to movement, access to care and allowed procedures began to ease in June 2020 and stayed the same to the end of that year. With rapid uptake of COVID-19 vaccination in January 2021 for all health care workers, access to health care returned to more normal levels in 2021. Washington State and in particular the Puget Sound Region may have had more rapid uptake of vaccinations and ability to open to care than other parts of the country.
On December 11, 2020, the Federal Drug Administration issued an Emergency Use Authorization for the Pfizer-BioNTech COVID-19 vaccine and for the Moderna COVID-19 vaccine on December 18, 2020. [7] Soon after vaccinations of essential workers in hospitals and clinics began. [8] As vaccination coverage and eligibility increased in 2021, vaccines became more readily available. Health care became more accessible by the first quarter (Q1) of 2021 and returned to more normal levels in 2021, the second year of the pandemic.
The COVID-19 pandemic created an unprecedented interruption in health care access. Our objective is to conduct a comprehensive evaluation of real-world experience at a tertiary cancer care center, evaluating pandemic restrictions impact on mammography screening, method of detection, stage at diagnosis, treatment and reasons for delays during 2020 and into 2021 when pandemic restrictions eased, including the relationship between these outcomes.
Methods
For our retrospective cohort study, we used patient data from our institutions’ breast cancer research registry database in the year prior to, during and after the pandemic restricted care delivery, 2019–2021. The database contains detailed information on method of diagnosis, patient characteristics, and stage at diagnosis. Clinical presentation characteristics including age, race, American Joint Committee Council on Cancer anatomic TNM stage, and method of detection by patient, mammography or other method were chart abstracted at time of diagnosis [9]. We included all women presenting with first primary breast cancer, biopsy confirmed and diagnosed in years 2019 to 2021 (N = 1797). Ten cases were dropped due to incomplete data (N = 1787). First primary breast cancer in the context of our study is when the patient has had no previous cancer diagnosis of any kind. We followed the STROBE observational study reporting guideline.
Incident BC cases were entered at time of diagnosis into the HIPAA compliant and IRB approved registry [IRB Study ID SWD39425-03]. This study was issued exemption status and approval by the Swedish Cancer Institute IRB program as the data is de-identified for analytic and study purposes. Information on each case demographics, diagnosis method, stage of disease, and treatment were included in initial data entry and as they occurred over time. Swedish Breast Care network radiology department utilization data was included in the IRB approval. All data used for analysis were de-identified at time of download prior to analysis.
At our institution, the dedicated Breast Cancer Research Registry Database has continuously entered BC patient information since 1995 by certified cancer registrars. The data is entered prospectively on all BC patients diagnosed or treated at our institution. Breast cancer detection method is a single variable in the registry with an option for detection method: patient (PtD), mammography (MamD), or other. Annual review of data integrity is conducted by the research staff.
Breast cancer detection method was obtained by medical record review at the time of diagnosis by a certified cancer registrar for each patient using the electronic medical chart and then input into the dedicated breast cancer research registry database. Mammography-detected breast cancer is disease discovered by routine mammography in the absence of complaints or known physical findings. Patient-detection was assigned if the patient detected breast symptoms, such as a palpable lump, pain, swelling, discharge, or bleeding prompting a clinic visit. Patients with self-detected tumors may have subsequent diagnostic mammograms or ultrasound done but are still labeled as a patient-detected breast cancer. ‘Other’ breast cancer detection includes physician detected or incidental findings from non-screening imaging for other complaints. If the detection method was ambiguous or incomplete, the tumor detection method was marked as unknown and those cases were excluded from the analysis. Diagnosis date for mammography detected BC is assigned on the date of positive biopsy after the BC is detected by mammography or confirmed by a diagnostic mammogram and ultrasound.
The institutional radiology department has billing utilization data from four imaging sites in the Swedish Breast Care network. Mammography utilization records are separated into screening and diagnostic imaging mammograms. Three of the four sites were temporarily closed on March 23, 2020 during the initial phase of the pandemic [3]. No mammography screening was conducted in April 2020 but diagnostic imaging for presenting non-screen detected cases continued. Restrictions on non-urgent procedures were reduced and mammography screening was resumed on a limited basis May 18, 2020 [3]. The utilization data is not linked to the Breast Cancer Registry Database used for this analysis but is at the same institution.
In our cohort study of breast cancer detection in the year prior to, during and after pandemic restricted care delivery, 2019–2021, we set the time periods to correlate with times of restricted access to health care. Pre-pandemic time 1 was all of 2019 and the first quarter (Q1) of 2020 before the State of Emergency Stay at Home orders were issued in Washington State effective March 23, 2020 [10]. Peak pandemic time 2 was the second to fourth quarter of 2020 when the nadir of screening and case diagnoses occurred. Pandemic 2021 time 3 was Q1-Q4 2021 after movement restrictions were lifted, protections were in place and vaccinations had begun. (Consort diagram Fig. 1)
Statistical analyses were done using de-identified data. IBM SPSS Statistics version 28 was used for all statistical analysis [11]. Statistical comparisons were done using the three time periods, (1) pre-pandemic time: 2019-Q1 2020, (2) peak-pandemic time: Q2-Q4 2020 and (3) pandemic 2021: Q1-Q4 2021 (Q = quarter). Pearson chi- square tests were used for bivariate analysis of dichotomous variables and analysis of variance was used for mean comparisons. All p values are two tailed with significance at the 0.05 level. Fishers exact testing was used in cases when a cell had less than 5 cases.
An adjusted binomial logistic regression model was used to calculate odds ratios and 95% confidence intervals for probability of outcome anatomic TNM stage 0/I. The model construction was informed by variables significant in the Pearson chi-square analyses (Table 1) including the three time periods pre-pandemic, peak pandemic and pandemic 2021. We are testing if the outcome of decreased stage 0/I is related to restricted mammography screening that was a non-essential service under Washington State mandates during the peak-pandemic time: Q2-Q4 2020. [12, 13]
Results
From 2019 to 2021, 1787 breast cancer cases were diagnosed at our institution. Compared to 2019 levels (N = 680), BC case volume was 22% lower in 2020 (N = 533) (p = .001) and 16% lower in 2021 (N = 574) (p = .012). (Fig. 2) Pre-pandemic case volumes did not differ from 2017 to 2019 [2017: n = 683, 2018: n = 687, 2019: n = 680].
From institutional radiology department utilization data, breast cancer case volume maximum decline was reached in April 2020 when mammography screening was suspended during the time of restricted access and cessation of non-urgent procedures in the second quarter of 2020. Diagnostic mammography continued to be done on a case-by-case basis. (Fig. 3) Screening mammograms declined 23% in 2020 (n = 43,885) compared to 2019 (n = 57,327) and increased 15% in 2021 from 2020 levels (n = 51,962) but not up to 2019 case volumes. From the same radiology utilization data, diagnostic mammograms declined 11% in 2020 and increased in 2021 but not up to 2019 levels [2019: n = 17,983, 2020: n = 15,949, 2021: n = 16,334]. (Fig. 4)
In our breast cancer cohort data analysis, age at diagnosis and race did not differ over the three time periods. Detection method shifted with mammography-detected breast cancer cases dropping in peak pandemic: Q2-Q4 2020 from 64 to 58% and patient-detected breast cancer increasing from 30 to 36% (p < .001) [pre-pandemic: MamD BC (n = 466), peak pandemic: MamD BC (n = 278); pre-pandemic: PtD BC (n = 221), peak pandemic: PtD BC (n = 171)]. In the cohort analysis, the largest change was in April and May 2020 (p < .001). (Fig. 5) Mammography detected cases with biopsy assigned diagnosis dates in April and May 2020 put them in months when no screening mammograms were done.
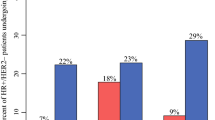
In pandemic time 2021 mammography detected breast cancer rebounded to 71%, 7% above the level observed prior to the pandemic [pre-pandemic: MamD BC = 64% (n = 466), pandemic 2021: MamD BC: 71% (n = 406) (p < .001)]. (Table 1) Concurrently, anatomic stage shifted with a drop in stage 0/I from pre-pandemic to peak-pandemic [pre-pandemic stage 0: 21% (n = 157), peak-pandemic stage 0: 16% (n = 78)] and stage I [pre-pandemic stage I: 40% (n = 291), peak-pandemic stage I: 38% (n = 181) (p < .001)]. Conversely in peak-pandemic, there was a relative higher proportion stage II and IV cancer from 28 to 33% stage II [pre-pandemic: n = 207, peak-pandemic: n = 157 (p < .001)], stage III no change and 2 to 4% stage IV [pre-pandemic: n = 12, peak-pandemic: n = 21 (p < .001)]. In pandemic time 2021, relative percent stage 0 increased to 22% of total (n = 128) and stage I to 45% of total (n = 260), but at overall case volume levels still below pre-pandemic time for each stage. In pandemic time 2021 stage II, III and IV percentages were below pre-pandemic levels [stage II 24% (n = 136), stage III 7% (n = 38), stage IV 2% (n = 12) (p < .001)]. (Fig. 6)
Hormone receptor status of diagnosed breast cancer cases did not change over time. Percent hormone therapy increased in peak-pandemic time but not significantly or numerically [pre-pandemic: n = 537 (73%), peak-pandemic: n = 367 (77%), p = .084)]. Neoadjuvant therapy, both chemo and hormone, increased from 13% (n = 99) pre-pandemic to 26% (n = 120) peak-pandemic and decreased to 16% in pandemic 2021 (n = 86) (p < .001). Twenty-three of the peak pandemic neoadjuvant therapy patients were given neoadjuvant hormone therapy due to COVID-19 related surgery delay (recorded in the chart). Chemotherapy treatment not including neoadjuvant therapy increased from 30% pre-pandemic (n = 222) to 36% peak-pandemic (n = 171) and back to pre-pandemic relative percentage in pandemic 2021 (29%, n = 166) (p = .047). (Table 1)
In a logistic regression model, outcome = stage 0/I BC, adjusted for age < 65/65+, race white/non-white and hormone receptor status present/absent, the odds of stage 0/I breast cancer diagnosis decreased 24% in the peak-pandemic time compared to pre-pandemic time [peak-pandemic: stage 0/I OR = 0.76, 95% CI: 0.60, 0.96, p = .021; age 65+: OR 1.46, 95% CI 1.18, 1.80, p < .001; race = white: OR = 0.85, 95% CI 0.69,1.05, p = .138; HR positive: OR = 2.53, 95% CI 1.86, 3.45, p < .001]. In the same model, pandemic 2021 odds of lower stage breast cancer diagnosis increased 34% compared to pre-pandemic [OR = 1.34, 95% CI: 1.06, 1.70, p = .014]. (Table 2)
Discussion
During the COVID-19 pandemic, health screening and care access were limited to varying degrees with treatment and diagnosis delays depending on local regulation and jurisdiction of health care delivery. In our study of the pandemic impact at our institution in Washington State, we reviewed how breast cancer diagnosis methods, stage at diagnosis, treatment and case volumes were affected by this unprecedented natural disaster-like event and the resulting regulations put in place to curtail COVID-19 spread. We observed a cascade of effects from pandemic related restrictions that affected screening, stage at diagnosis, case volumes, and treatment delays with alternative therapy options enacted in 2020.
In the second quarter of 2020 the impact of COVID-19 closures and restrictions began to take effect at our institution and there was a decrease in breast cancer case volume. In April 2020, mammography screening was stopped while diagnostic mammograms continued on a limited basis dictated by case prioritization directives. During the peak pandemic time, Q2-Q4 of 2020, case volume declined and there was a numeric and proportional decrease in stage 0/I BC relative to stage II-IV BC due to the discontinuation and restriction of non-essential screening in Washington State during the peak pandemic period. In 2021 when mammography screening became more available, the trend reversed to more stage 0 and I, mammography detected breast cancer diagnosed but number of cases were still below 2019 pre-pandemic levels. Neoadjuvant therapy increased during peak-pandemic Q2-Q4 2020 when pandemic restrictions limited care access causing surgery delays and alternative therapies were made available. The most common COVID-19 related alternative treatment observed was pre-operative hormone therapy given to lower stage hormone receptor positive cases due to restrictions on non-urgent surgery. Other treatments affected or delayed were chemotherapy, radiation therapy and treatment planning.
Higher stage II-IV breast cancer diagnosis did not change significantly during the pandemic with a relative proportional increase due to declining number of mammography detected cases stage 0 and I in the peak pandemic time Q2-Q4 2020. In 2021, the relative proportion of stage II-IV BC declined when mammography screening was available and number of stage 0 and I breast cancers increased. In a comparison of only stage II-IV breast cancers, number and proportion of stage II-IV BC did not change significantly over the 3 time periods (data not presented).
Some preliminary studies of the effects of COVID-19 pandemic restrictions on health care access and/or screening for breast cancer have been reported. Decreased mammography screening occurred during the pandemic onset in 2020 [14,15,16,17]. Two studies reported higher proportion of higher stage or symptomatic disease in the 2020 shelter-in-place time period [18, 19]. One study reported increased neoadjuvant therapy [20].
Decline in diagnosed breast cancer case volumes was observed in the US in association with the COVID-19 pandemic in 2020 [21]. In Turkey, a study of a single institution found the volume of breast surgery declined in 2020 [22]. Velazquez et al. tracked screening mammograms at their institution during the 2019–2020 time period and observed a sharp decline in screening mammograms beginning in March 2020 with an increasing number of missed appointments and a slow increase later in 2020 but the number of screening mammograms remained below 2019 baseline levels [14]. In a large study of mammography utilization through 2021, a 40% decrease in mammography was observed nationally in 2020, with rates remaining below normal into 2021, but screening and diagnostic mammography were not differentiated [23]. Similarly, Doan et al. in a study of Medicare enrollee utilization of breast cancer screening found profound decreases in screening starting in March 2020 with continued monthly rate decreases related to increases in national COVID-19 infection rates [24]. Decreases in Medicare cancer screening observed did not resolve after initial pandemic surges.
In the Flanders region of Belgium, breast cancer screening invitation coverage dropped 10% in 2020 but the backlog of invitations was largely resolved in 2021 [25]. The authors concluded a minimal influence on willingness to screen existed but coverage of screening may have been impacted. Duffy et al. assessed the impact of the temporary COVID-19-related cessation of population screening on breast cancer deaths in England. They projected between 148 and 687 additional breast cancer deaths could occur depending on how quickly delays were caught up [26].
In 2020, Guth et al. observed fewer mammography detected breast cancers, more breast cancer detected by self-exam which were palpable on presentation, and fewer DCIS cases between 4/1/2020 and 3/31/2021 [27]. During the time when only essential surgery was allowed, more patients were treated with neoadjuvant chemotherapy and neoadjuvant hormonal therapy. They did not observe an increase in higher stage III and IV breast cancer and observed a decrease in radiation therapy. Zhou et al. observed a significantly lower number of stage I BC and higher amount of stage IV in 2020 after the start of the pandemic [28]. Trojanowski et al. observed a stage shift in 2021 with stage II BC more frequent than stage I and a significant increase in stage III [29].
On April 1, 2020, The American Society of Breast Surgeons issued a guide for use of neoadjuvant endocrine therapy related to surgical delays during the pandemic to assist with triaging breast cancer patients [30]. In 2020, authors Marti et al. and Thompson et al. discussed priorities and options for estrogen receptor positive breast cancer management due to surgical delays [31, 32]. Tonneson et al. observed a 10% increase in neoadjuvant endocrine therapy during the COVID-19 restricted access to care [33]. Habbous et al. observed a 20% increase in neoadjuvant systemic therapy during COVID-19-related care access, both hormonal and chemotherapy [34].
In our cohort, the percentage mammography detected breast cancer cases rebounded to above 2019 levels with a shift to earlier stage cancers very quickly in 2021, although actual number of cases remained below 2019 levels. These findings indicate the impact from delayed screening may have had minimal effect on compliant screeners who quickly made up their missed appointments. The continued lower than usual case volume of both screening mammograms and diagnosed BC cases overall indicate a missing portion of the potential screened population exists.
The 24% decrease in early stage 0 and I breast cancer in peak-pandemic time Q2-Q4 2020 was related to stoppage of mammography screening and limited access in 2020 followed by catch up screening in 2021 with early stage 0 and I breast cancer 34% higher than pre-pandemic year 2019/Q1 2020. However, mammography screening in the United States relies on opportunistic or invitational mammography screening depending on where one receives care, whether the care delivery organization has a reminder system, and type of insurance if one has health insurance. Some guidelines are based on United States Preventive Services Task Force, the American Cancer Society and other organizations recommendations unlike countries with singular organized screening programs [35,36,37,38]. Our institutional radiology department uses American College of Radiology (ACR) guidelines which recommend annual screening starting at age 40 [39]. Screening is therefore predicated on self-initiation of screening mammography or prompting by a care provider or health care system. The continued lower volume of diagnosed breast cancer cases and screening mammograms into 2021 is concerning.
Strengths and limitations
Institutional level radiology utilization data provided timing of changes in mammography screening. Our institution’s mammography utilization data is not linked to the patient level data used in the registry cohort. For the cohort, screening mammography method of detection is obtained from physician chart notes. The level of detailed data collected on each patient in the registry database allowed conduct of a comprehensive evaluation of multiple related impacts of delayed and restricted access to care during the pandemic, not available in larger national databases of utilization or insurance claims data. Even though the number of cases in our study is not as large as a national database, the full extent of diagnosis, breast cancer characteristics, and treatment available in the registry enabled a more complete picture of COVID-19 pandemic breast cancer care.
Conclusions
Future tracking of breast cancer cases with treatment and diagnosis changes may be useful for guidelines in cases of natural disaster if triage of cases becomes essential again. Important lessons from the COVID-19 pandemic effect are the creation of essential surgery definitions, alternative therapy for surgical delays, and accelerated return to routine screening to remove backlogs of screening. [40] Return to previous levels of screening mammography will require population-based patient reconnection to usual care providers and related systems with scheduled reminder systems as well as return to previous staffing levels and care availability.
Data Availability
The data underlying this article cannot be shared publicly owing to the need to protect the privacy of the study participants.
References
Holshue ML, DeBolt C, Lundquist S et al (2020) Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med 382(10):929–936. https://doi.org/10.1056/NEJMoa2001191
Governor of Washington (2022) Proclamation by the Governor amending proclamation 20 – 05. Public Meetings Act and Public Records Act. https://www.governor.wa.gov/sites/default/files/proclamations/20-28.2%20-%20COVID-19%20Open%20Govt%20Waivers%20Ext%20%28tmp%29.pdf
Emergency Proclamation by the governor amending proclamations 20-05, and 20-24. et seq. 20-24.3 Restrictions on Non-Urgent Medical Procedures. https://wa.gov/. Accessed June 15 2022
The American Society of Breast Surgeons and The American College of Radiology (2021) Joint statement on breast screening exams during the Covid-19 pandemic. ASBrS and ACR Joint Statement on Breast Screening Exams During the COVID-19 Pandemic (breastsurgeons.org). Accessed December 2021
Dietz JR, Moran MS, Isakoff SJ et al (2020) Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. Breast Cancer Res Treat 181(3):487–497. https://doi.org/10.1007/s10549-020-05644-z
Sprague BL, O’Meara ES, Lee CI et al (2021) Prioritizing breast imaging services during the COVID pandemic: a survey of breast imaging facilities within the breast Cancer Surveillance Consortium. Prev Med 151:106540. https://doi.org/10.1016/j.ypmed.2021.106540
https://www.cdc.gov/museum/timeline/covid19.html. Accessed March 20, 2023
https://doh.wa.gov/sites/default/files/legacy/Documents/1600/coronavirus//VaccinationPhasesInfographic.pdf. Accessed March 20, 2023
Kalli S, Semine A, Cohen S, Naber SP, Makim SS, Bahl M (2018) American Joint Committee on Cancer’s staging system for breast Cancer, Eighth Edition: what the radiologist needs to know. Radiographics 38(7):1921–1933. https://doi.org/10.1148/rg.2018180056
Proclamation by the Governor Amending Proclamation 20 – 05 20–25 Stay Home -Stay Healthy, March (2020) 24, 20–25 Coronovirus Stay Safe-Stay Healthy (tmp) (002).pdf (wa.gov). Accessed June 15 2022
IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY:IBM Corp
Malmgren JA, Parikh J, Atwood MK, Kaplan HG (2012) Impact of mammography detection on the course of breast cancer in women aged 40–49 years. Radiology 262(3):797–806. https://doi.org/10.1148/radiol.11111734
Onitilo AA, Engel JM, Liang H, Stankowski RV, Miskowiak DA, Broton M, Doi SA (2013) Mammography utilization: patient characteristics and breast Cancer stage at diagnosis. AJR 201(5):1057–1063. https://doi.org/10.2214/AJR.13.10733
Velazquez AI, Hayward JH, Gregory G, Dixit N (2021) Trends in breast cancer screening in a safety-net hospital during the COVID-19 pandemic. JAMA Netw Open 4(8):e2119929. https://doi.org/10.1001/jamanetworkopen.2021.19929
Sprague BL, Lowry KP, Miglioretti DL, Alsheik N, Bowles EJA, Tosteson ANA et al (2021) Changes in mammography use by women’s characteristics during the first 5 months of the COVID-19 pandemic. J Natl Cancer Inst 113(9):djab045. https://doi.org/10.1093/jnci/djab045
Teglia F, Angelini M, Astolfi L, Casolari G, Boffetta P (2022) Global association of COVID-19 pandemic measures with cancer screening: a systematic review and meta-analysis. JAMA Oncol 8(9):1287–1293. https://doi.org/10.1001/jamaoncol.2022.2617
Chen RC, Haynes K, Du S, Barron J, Katz AJ (2021) Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol 7(6):878–884. https://doi.org/10.1001/jamaoncol.2021.0884
Tang A, Neeman E, Vuong B, Arasu VA, Liu R, Kuehner GE, Savitz AC, Lyon LL, Anshu P, Seaward SA, Patel MD, Habel LA, Kushi LH, Mentakis M, Thomas ES, Kolevska T, Chang SB, Permanente Medical Group Breast Research Collaborative (2022) Care in the time of COVID-19: impact on the diagnosis and treatment of breast cancer in a large, integrated health care system. Breast Cancer Res Treat 191(3):665–675. https://doi.org/10.1007/s10549-021-06468-1
Lloyd MR, Stephens SJ, Hong JC, James TA, Mehta T, Recht A, Spiegel D (2021) The impact of COVID-19 on breast cancer stage at diagnosis. J Clin Onc 39(15):suppl528. https://doi.org/10.1200/JCO.2021.39.15_suppl.528
Caswell-Jin JL, Shafaee MN, Xiao L, Liu M, John EM, Bondy ML, Kurian AW (2022) Breast cancer diagnosis and treatment during the COVID-19 pandemic in a nationwide, insured population. Breast Cancer Res Treat 194(2):475–482. https://doi.org/10.1007/s10549-022-06634-z Epub 2022 May 27
Kaufman HW, Chen Z, Niles JK, Fesko YA (2021) Changes in newly identified cancer among US patients from before COVID-19 through the first full year of the pandemic. JAMA Netw Open 4(8):e2125681. https://doi.org/10.1001/jamanetworkopen.2021.25681
Kara H, Arikan AE, Dulgeroglu O, Tutar B, Tokat F, Uras C (2022) Has the COVID-19 pandemic affected breast cancer stage and surgical volume? Front Surg 7(9):811108. https://doi.org/10.3389/fsurg.2022.811108
Oakes AH, Boyce K, Patton C, Jain S (2023) Rates of routine cancer screening and diagnosis before vs after the COVID-19 pandemic. JAMA Oncol 9(1):145–146. https://doi.org/10.1001/jamaoncol.2022.5481
Doan C, Li S, Goodwin JS (2023) Breast and lung cancer screening among medicare enrollees during the COVID-19 pandemic. JAMA Netw Open 6(2):e2255589. https://doi.org/10.1001/jamanetworkopen.2022.55589
Jidkova S, Hoeck S, Kellen E, le Cessie S, Goossens MC (2022) Flemish population-based cancer screening programs: impact of COVID-19 related shutdown on short-term key performance indicators. BMC Cancer 18(1):183. https://doi.org/10.1186/s12885-022-09292-y
Duffy SW, Seedat F, Kearins O, Press M, Walton J, Myles J, Vulkan D, Sharma N, Mackie A (2022) The projected impact of the COVID-19 lockdown on breast cancer deaths in England due to the cessation of population screening: a national estimation. Br J Cancer 126:1355–1361. https://doi.org/10.1038/s41416-022-01714-9
Guth AA, Diskin B, Schnabel F, Pourkey N, Axelrod D, Shapiro R (2022) Changes in breast cancer presentation during Covid-19: Experience in an Urban Academic Center [abstract]. In: Proceedings of the 2021 San Antonio Breast Cancer Symposium; 2021 Dec 7–10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 82(4 Suppl):Abstract nr P2-03-01. https://doi.org/10.1158/1538-7445.SABCS21-P2-03-01
Zhou JZ, Kane S, Ramsey C et al (2021) Comparison of early- and late-stage breast and colorectal cancer diagnoses during vs before the COVID-19 pandemic. JAMA Netw Open 5(2):e2148581. https://doi.org/10.1001/jamanetworkopen.2021.48581
Trojanowski M, Radomyski P, Matuszewski K, Litwiniuk M, Wierzchosławska E, Kycler W (2022) Impact of the COVID-19 pandemic on breast cancer stage at diagnosis in a regional cancer center in Poland between 2019 and 2021. J Pers Med 12(9):1486. https://doi.org/10.3390/jpm12091486
ASBrS Resource (2022) Guide to Endocrine Therapy in the COVID-19 Pandemic (breastsurgeons.org). Issued April 1, 2020. Accessed November 15, 2022
Marti C, Sánchez-Méndez JI (2020) Neoadjuvant endocrine therapy for luminal breast cancer treatment: a first-choice alternative in times of crisis, such as the COVID-19 pandemic. ecancer 14:1027. https://doi.org/10.3332/ecancer.2020.1027
Thompson CK, Lee MK, Baker JL, Attai DJ, DiNome ML (2020) Taking a second look at neoadjuvant endocrine therapy for the treatment of early stage estrogen receptor positive breast cancer during the COVID-19 outbreak. Ann Surg 272(2):e96–e97. https://doi.org/10.1097/SLA.0000000000004027
Tonneson JE, Hoskin TL, Day CN, Durgan DM, Dilaveri CA, Boughey JC (2022) Impact of the COVID-19 pandemic on breast cancer stage at diagnosis, presentation, and patient management. Ann Surg Oncol 29:2231–2239. https://doi.org/10.1245/s10434-021-11088-6
Habbous S, Tai X, Beca JM, Arias J, Raphael MJ, Parmar A et al (2022) Comparison of use of neoadjuvant systemic treatment for breast cancer and short-term outcomes before vs during the COVID-19 era in Ontario, Canada. JAMA Netw Open 5(8):e2225118. https://doi.org/10.1001/jamanetworkopen.2022.25118
Final Recommendation Statement. Breast Cancer: Screening. January 11 (2016) http://uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening. Accessed November 15, 2022
American Cancer Society Guidelines for the Early Detection of Cancer (2022) www.cancer.org/healthy/find-cancer-early/cancer-screening-guidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2022
Hofvind S, Vacek PM, Skelly J, Weaver DL, Geller BM (2008) Comparing screening mammography for early breast cancer detection in Vermont and Norway. J Natl Cancer Inst 100(15):1082–1091. https://doi.org/10.1093/jnci/djn224
Smith-Bindman R, Chu PW, Miglioretti DL, Sickles EA, Blands R, Ballard-Barbash R et al (2003) Comparison of screening mammography in the United States and the United Kingdom. JAMA 290:2129–2137. https://doi.org/10.1258/0969141053279130
Monticciolo DL, Newell MS, Hendrick RE, Helvie MA, Moy L, Monsees B, Kopans DB, Eby PR, Sickles EA (2017) Breast Cancer screening for average-risk women: recommendations from the ACR Commission on breast imaging. J Am Coll Radiol 14(9):1137–1143. https://doi.org/10.1016/j.jacr.2017.06.001
Mattingly AS, Eddington HS, Rose L, Morris AM, Trickey AW, Cullen MR, Wren SM (2023) Defining essential surgery in the US during the COVID-19 pandemic response. JAMA Surg 158(1):99–100. https://doi.org/10.1001/jamasurg.2022.3944
Funding
This study was supported the Kaplan Cancer Research Fund.
Author information
Authors and Affiliations
Contributions
Both Drs. Judith Malmgren and Henry Kaplan contributed to the study conception and design. Material preparation and data collection were performed by Mary Atwood, Dr. Paula Hallam and Laura Roberts, data analysis was performed by Dr. Judith Malmgren and Boya Guo. The first draft of the manuscript was written by Dr. Judith Malmgren and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is an observational cohort study using de-identified data. The institutional review board confirmed that no ethical approval is required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Malmgren, J.A., Guo, B., Atwood, M.K. et al. COVID-19 related change in breast cancer diagnosis, stage, treatment, and case volume: 2019–2021. Breast Cancer Res Treat 202, 105–115 (2023). https://doi.org/10.1007/s10549-023-06962-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06962-8