Abstract
Purpose
This study aimed to compare the prognosis in terms of disease-free survival (DFS) in three populations of women with breast cancer (BC) treated with neoadjuvant systemic treatment (NAST) in which axillary lymph node dissection (ALND) was performed based on different total tumor load (TTL) thresholds in the sentinel nodes.
Methods
This was an observational, retrospective study carried out in three Spanish centers. Data from patients with infiltrating BC who underwent BC surgery after NAST and intraoperative sentinel lymph node biopsy (SLNB) performed by One Step Nucleic acid Amplification (OSNA) technique during 2017 and 2018 were analyzed. ALND was performed according to the protocol of each center, based on three different TTL cut-offs (TTL > 250, TTL > 5000, and TTL > 15,000 CK19-mRNA copies/μL for centers 1, 2, and 3, respectively).
Results
A total of 157 BC patients were included in the study. No significant differences in DFS were observed between centers (Hazard ratio [HR] center 2 vs 1: 0.77; p = 0.707; HR center 3 vs 1: 0.83; p = 0.799). Patients with ALND had a shorter DFS (HR 2.43; p = 0.136), albeit not statistically significant. Patients with a triple negative subtype had a worse prognosis than those with other molecular subtypes (HR 2.82; p = 0.056).
Conclusion
No significant differences in DFS were observed between three centers with different surgical approaches to ALND based on different TTL cut-offs in patients with BC after NAST. These results suggest that restricting ALND to those patients with TTL ≥ 15,000 copies/μL is a reliable approximation, avoiding unnecessary morbidities caused by ALND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is one of the main life-threatening neoplasms in women worldwide [1]. In Spain, BC is the leading cause of cancer and cancer-related death in women [2]. The use of neoadjuvant systemic treatment (NAST) as a preoperative treatment modality is currently being extended to earlier stages as it offers a wide range of benefits, including the opportunity to further de-escalate the surgical management of the axilla [3,4,5,6]. However, there is still a lack of consensus regarding the post-treatment surgical approach to axillary lymph node dissection (ALND).
Contrary to ALND, sentinel lymph node biopsy (SLNB) is a minimally invasive procedure that allows accurate axillary nodal staging with less morbidity. It has been established as the gold standard for pathological evaluation of the axilla in patients with operable BC and clinically negative axilla. SLNB diagnostic performance by conventional histopathology following NAST remains controversial, since this treatment may affect the lymphatic drainage and causes tissue alterations, limiting the nodal histological evaluation [7,8,9]. Hence, the use of a molecular method may be more advisable.
One Step Nucleic acid Amplification (OSNA) is a molecular technique that allows the detection of the number of copies of messenger Ribonucleic Acid (mRNA) of cytokeratin 19 (CK19) present in the sentinel lymph node (SLN). CK19 is a membrane protein expressed by most breast carcinomas even after NAST, constituting a good target to detect BC metastasis [10, 11].The calculation of the total tumor load (TTL) based on CK19 mRNA copy number is a highly sensitive and specific method for the detection of micro and macrometastasis of lymph nodes (LNs) [12, 13]. TTL is a quantitative index that provides information on the metastatic load present in the LNs, in an objective manner. Furthermore, the OSNA method is an automated and reproducible approach to calculate TTL using metastatic load information [14]. Therefore, its use could provide more sensitive information regarding the response to NAST in the LNs compared with conventional histological examination, limiting overtreatment or unnecessary ALND and adding more precision to the axillary surgical management [7, 15].
The prognostic value of TTL, measured by OSNA, was previously demonstrated in a population of BC patients that did not receive NAST (PLUTTO study), confirming the clinical value of the TTL. Furthermore, this study established a correlation of TTL with disease-free survival (DFS) and defined two risk groups (TTL < 25,000 copies/μL: low-risk and TTL ≥ 25,000 copies/μL: high-risk) [16]. This prognostic value of TTL, measured using the OSNA technique, was also demonstrated for BC patients after NAST in the NEOVATTL study [17], showing a clear decrease in survival at TTL ≥ 25,000 copies/μL. Although this prognostic value of TTL seems to be valid in the population with and without NAST, the subsequent surgical strategy to the axillary approach, based on the results of the SLN assay and TTL values, varies widely in clinical practice. In this regard, clinical guidelines recommend performing ALND in presence of any nodal involvement (even isolated-tumor cells and/or micrometastases) [18]. Moreover, the NEOVATTL study showed that TTL values > 15,000 copies/μL were predictive of LN involvement after intraoperative OSNA assay in patients with BC after NAST.
Despite the predictive and prognostic value of TTL derived from intra-operative OSNA assay in SLN of BC patients after NAST was demonstrated in the NEOVATTL study, evidence is still scarce. These data could be relevant to develop protocols using TTL cut-off points to guide the surgical strategy regarding the axillary approach in these patients. This retrospective study aimed to compare the prognosis in terms of disease-free survival (DFS), in three populations of women with BC treated with NAST in which TTL values were used as criteria to perform ALND.
Materials and methods
Study design and population
This was an observational, retrospective, multicenter study of clinicopathologic data registry analysis and follow-up of women with infiltrating BC who had undergone BC surgery and SLNB. Patients with at least 3 years of follow-up who received NAST followed by SLNB with OSNA technique during 2017 and 2018 were included in the study. Patients under 18 years of age, with carcinoma in situ or other malignant neoplasms, and those considered unsuitable for the study by the investigator were excluded, such as those with no migration of the tracer used in the SLNB during surgery, precluding detection of the SLN, and those lost to follow-up.
This study was carried out in three Spanish centers. Surgeons performed ALND according to the protocol of each center, based on three different TTL cut-offs. Thus, patients with TTL > 250 (center 1), TTL > 5000 (center 2), TTL > 15,000 (center 3) CK19 mRNA copies/μL were selected for ALND in each center. Data from those patients meeting the eligibility criteria of the study were collected from the digital medical records in September–October 2021. The investigator included each patient’s data in the case report form in an anonymized manner.
This study was performed after approval from the Comité Ético provincial de Andalucía, PEIBA (Ethics Committee of Andalucía) and the Instituto de Investigación Biomédica de Salamanca (IBSAL) (Salamanca Biomedical Research Institute). Furthermore, it was developed following the ethical principles originating from the latest version of the Declaration of Helsinki accepted by local authorities and which are in line with Good Clinical Practice (GCP) and the requirements of current Spanish regulations.
Endpoints, variables, and assessments
The main objective of this study was to compare the prognosis in terms of DFS in three populations of women with BC treated with NAST (considering DFS as the time until evidence of disease recurrence or progression was found). The three populations differed in the criteria for the indication for ALND, which was TTL > 250, TTL > 5000, and TTL > 15,000 CK19 mRNA copies/μL (measured using the OSNA technique) according to each center's protocol. The secondary objectives were to compare the prognosis after NAST in the three populations (i.e., with different criteria for ALND indication) according to tumor subtype and to calculate the percentage of unnecessary ALND in the three populations, as the weighted mean of ALND with no positive LNs at each center.
In addition to variables associated with the primary objective (i.e., tumor recurrence location and date) and demographic characteristics (i.e., age), previous tumor features and type of neoadjuvant, surgical, and adjuvant treatments were collected. Tumor characteristics included TNM classification, histological type, grade, hormonal receptors, HER2 status, Ki67 (% of positive cells), and molecular subtype. SLNB analysis before neoadjuvant treatment (yes/no), type of neoadjuvant treatment (chemotherapy/hormonotherapy), and radiological evaluation of response to NAST (in breast and axilla) were also recorded. Surgery characteristics evaluated were the type of breast surgery (conservative/radical), data related to SLNB (OSNA evaluation, tumor load per node analyzed, and TTL), and ALND (yes/no; non-sentinel metastatic nodes/non-sentinel nodes removed). Adjuvant strategies were additionally collected, including type of treatment: chemotherapy (yes/no), hormone (yes/no), and radiotherapy (yes/no). Finally, recurrence disease (site and date) and exitus (yes/no; date) was also considered.
Statistical analysis
A descriptive analysis was performed for all variables of interest using centralization and dispersion parameters. Quantitative variables were described using the mean and the standard deviation (SD), and the median (P50) together with the 25th and 75th percentiles (P25–P75) as well as the minimum and maximum values. Qualitative variables were described using the absolute frequencies and percentages together with the 95% confidence interval (95% CI).
To achieve the main objective, the DFS of the sample was described using the Kaplan–Meier analysis, considering the recurrence or progression of the disease as an event. Furthermore, Kaplan–Meier curves were compared between different risk groups. Differences between these groups were evaluated by hazard ratio (HR) (95% CI). Statistical significance (p value) was computed using log-rank test.
For the other objectives, univariate Cox regression analyses were performed to estimate the HR of recurrence/progression associated with each of the potential predictive variables of interest, including TTL cut-off for ALND in each center, ALND, and molecular subtype (independent variables) and time to event as the dependent variable. Center 1 and no ALND were used as references for the calculation of the HRs when comparisons were made between centers and ALND (yes/no).
All analyses were conducted with the R language (version 4.2.1) installed on windows 10, using functions implemented in the Survival and survminer packages for Cox model fitting and evaluation.
Results
Characteristics of the study population
A total of 157 BC patients were included in the study, of which 28 belonged to center 1, 72 to center 2, and 57 to center 3. The mean age of the patients was 59 (±SD 13.1) years and was similar between the different centers. Most of the patients had a cT2N0 and were diagnosed with invasive breast carcinoma, not otherwise specified (NOS) histologic type (Table 1). The hormonal receptors and HER2 status, the molecular subtype and data related to neoadjuvant treatment are included in Tables S1 and S2 (found in Online Resource 1). There were no differences in TTL values according to molecular subtype (other vs. triple negative).
Surgery characteristics and TTL
Regarding the characteristics of the surgery, most patients underwent a conservative surgical approach. A total of 136 patients did not undergo ALND. The remaining 21 patients underwent ALND (7 from center 1, 8 from center 2, and 6 from center 3). Most patients (belonging to centers 2 and 3) lacked metastatic non-sentinel node compromise. In centers 1 and 2, 14.3% and 25% of the 7 and 8 patients with ALND, respectively, presented negative lymphadenectomy, which could have been avoided by applying the 15,000 copies cut-off criterium (Table S4 found in Online Resource 1). Based on the primary outcome, the percentage of unnecessary ALND, defined as negative ALND in patients with < 15,000 copies/μL, in the three centers was 14.3% (Table 2).
DFS according to center and ALND
A total of 14 patients relapsed, with a similar distribution between centers and without a predominant body location. Additionally, seven patients died (Table 3). No significant differences were found in DFS according to the type of surgery (conservative or radical) (HR 1.30 95% CI 0.43–3.87; p = 0.642). Regarding TTL values of non-relapsing patients, 74.8% had a TTL value under 250 copies/μL, and 7.0% had a TTL value above 15,000 copies/μL (Table 4).
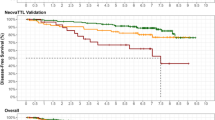
Regarding DFS (main objective), no significant differences were observed between centers, with different TTL cut-off points per center (HR center 2 vs 1: 0.77; 95% confidence interval [95% CI] 0.19–3.07, HR center 3 vs 1: 0.83 95% CI 0.20–3.48; p = 0.707 and 0.799 respectively) (Fig. 1).
Regarding DFS according to ALND, patients with ALND had a shorter DFS, although differences were not statistically significant (HR 2.43, 95% CI 0.76–7.79; p = 0.136) (Fig. 2). In those patients with no ALND performed, no significant differences were observed between centers (HR center 2 vs 1: 0.77, 95% CI 0.77–4.00 p = 0.758; HR center 3 vs 1: 0.58, 95% CI 0.58–3.50 p = 0.555) (Fig. 3). Analysis of DFS stratified by center according to ALND, showed no differences between centers (HR center 2 vs 1: 0.76, 95% CI 0.13–4.35 p = 0.756; HR center 3 vs 1: 0.69, 95% CI 0.10–4.80 p = 0.706) (Fig. 4). Patients without ALND were insufficient to establish a relationship between DFS and center. Within these patients, less than 10% had recurrence, with no significant differences between centers (Table S5).
The evaluation of the secondary objective of DFS according to the molecular subtype of BC showed that patients with a triple negative subtype have a worse prognosis than those with other molecular subtypes (HR 2.82; 95% CI 0.97–8.16; p = 0.056) (Fig. 5).
Discussion
A consensus about axillary approach in patients with BC after neoadjuvant therapy, especially in early clinical stages, is still missing. The SNB has been accepted as a good tool for stablishing the axillary status, with the aim to avoid unnecessary ALND, but SLN histological assay is limited due to tissue alterations resulting from the previous systemic treatment. OSNA analysis may avoid these tissue limitations and provide a quantitative result.
Although previous studies have demonstrated the value of TTL as a prognostic marker for non-sentinel node disease in patients with BC with or without NAST, the cut-off to indicate ALND differs significantly among groups, especially after NAST [16, 17].
This retrospective study compared the outcomes (i.e., DFS) of BC patients who, after NAST, underwent BC surgery with intraoperative SLNB and analysis with the OSNA technique in three centers, with different ALND indication criteria based on TTL values. No significant differences in DFS were observed between centers performing ALND at TTL > 250, > 5000, and > 15,000 CK19 mRNA copies/μL.
The OSNA method quantifies the number of CK 19 mRNA copies/μL, classifying SLNBs with TTL < 250 copies/μL as negative, with 250–5000 copies/μL as micrometastasis, and with > 5000 copies/μL as macrometastasis [22]. In this study, patients underwent ALND following the criteria of each participating center. According to the definitions of the OSNA method and center criteria, all patients with micrometastases underwent ALND in center 1, while only those with macrometastases underwent ALND in centers 2 and 3, with different cut-off between both centers: > 5000 copies/μL in center 2 and > 15,000 copies/μL in center 3. The decision of the study centers to perform ALND according to TTL and not number of affected SLNs is in line with recent reports describing TTL in SLNs as an independent predictor of axillary involvement and showing a higher predictive ability of SLN involvement compared to the number of affected SLNs [14, 20, 23,24,25,26,27].
Few studies have provided prognostic information about the relationships between TTL in SLNs and prognosis (i.e., DFS) in patients with BC who have received prior NAST. NEOVATTL study was the first published study that assessed the predictive and prognostic value of TTL derived from molecular analysis of the SLN in BC patients after neoadjuvant systemic therapy. This study demonstrated that a TTL > 15,000 copies/μL predicted non-sentinel axillary affectation and reported that TTL > 25,000 copies/μL was associated with a higher risk of disease recurrence in 316 patients with a mean TTL of (42,314 ± 248,208) [17]. However, no significant difference in DFS prognosis between patients with a TTL of < 250 copies/μL versus ≥ 250–25,000 copies/μL was observed. With these findings, Vieites et al. suggested that small metastases (> 250 and ≤ 25,000 copies/μL) detected by OSNA have similar prognostic value to negative nodes, with clinical outcomes no worse than a patient with negative nodes. In this study evaluating 157 patients, the results in terms of DFS were similar between centers with TTL cut-off values for ALND of > 250, > 5000, and > 15,000 CK19 mRNA copies/μL. Based on these results, axillary management could be conservative in patients with TTL lower than 15,000 copies/μL, avoiding unnecessary ALND. Considering the previously identified TTL cut-off of 25,000 copies/μL to predict disease recurrence, it is possible that even more conservative approaches (i.e., performing ALND at > 15,000 and < 25,000 copies/μL) may have resulted in similar outcomes.
Regarding secondary objectives, concerning to the relationship between BC subtype and DFS, the results of this study have demonstrated that patients with a triple negative subtype had a worse prognosis in terms of DFS than those with other BC molecular subtypes. These findings are consistent with current scientific evidence describing a relationship between triple-negative BC and lower survival compared to the other BC subtypes [27,28,29,30].
Compared to the NEOVATTL study, the distribution of the percentage of patients with recurrences within the different cut-off groups was similar. Thus, in the NEOVATTL study, disease recurrence rates were 51.3% in the group of patients with TTL of < 250 copies/μL; 23.1% in those with TTL between 250 and 25,000 copies/μL, and 25.6% in the patients with > 25,000 copies/μL, with an overall disease recurrence rate of 12.4% (39 patients) [17]. In this study, also half of the patients with BC recurrence had TTL < 250 copies/μL with an overall disease recurrence rate of 8.9% (14 patients). Those results suggest that other clinicopathological factors, such as the surrogate molecular subtype, may influence the risk of recurrence.
Although ALND has been associated with significant morbidity (including the risk of developing lymphedema of the upper limb, paresthesia, pain, and restriction of motion of the shoulder girdle), the surgical strategy to the axillary approach, depending on the results of the SLN study, continues to vary widely in clinical practice. Thus, while some follow the criterion proposed by the ACOSOG-Z0011 Trial, according to which ALND could be avoided in patients with T1-2 BC and one or two positive SLNs, others use the value of the TTL detected in the SLN(s) after analysis with OSNA [31]. Furthermore, according to some authors, this method adds accuracy to the node assay after NAST because it measures CK19 derived from viable tumor cells, providing accurate information about residual tumor cells [21]. In this second case, even though the OSNA method is a standardized technique, there is no agreement between the groups on the cut-off point from which ALND should be performed. In this regard, the main objective of this study was to compare prognosis among three populations of BC patients after NAST with different surgical approaches according to TTL levels and no significant differences in DFS were found. These findings suggest that restricting ALND to those patients with TTL > 15,000 CK mRNA copies/μL is a reliable approximation avoiding unnecessary morbidities caused by ALND.
The limitations of this study are attributable to the retrospective nature of its design, including the risk of patient selection bias. In addition to the limited number of patients in this study and the presence of variability in patient follow-up times, the low number of patients with ALND (n = 21) and the few recorded events may have influenced the analysis of DFS according to ALND. Finally, the uneven recruitment between centers resulted in an uneven distribution of patients between groups.
Despite these limitations, the results of this study, showing similar outcomes irrespective of the axillary approach based on different TTL cut-off values, could guide decision-making regarding ALND and breast-conserving surgery in BC patients who have received NAST. Prospective and multicenter randomized controlled studies are necessary to determine the predictive value of TTL for the diagnosis of non-SLN metastases and establish cut-off points that could guide the surgical strategy in terms of the axillary approach after NAST in patients with BC.
Conclusion
In this study, no significant differences in DFS were observed between three centers with different surgical approaches to ALND based on different TTL cut-offs obtained in the SNs assay, in patients with BC after NAST. These results suggest that restricting ALND to those patients with TTL ≥ 15,000 CK mRNA copies/μl is a reliable approximation avoiding unnecessary morbidities caused by ALND.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Sociedad Española de Oncología Médica (SEOM) LAS CIFRAS DEL CANCER EN ESPAÑA 2022. https://seom.org/images/LAS_CIFRAS_DEL_CANCER_EN_ESPANA_2022.pdf. Accessed 14 Oct 2022
Li X, Wang M, Wang M et al (2019) Predictive and prognostic roles of pathological indicators for patients with breast cancer on neoadjuvant chemotherapy. J Breast Cancer 22:497. https://doi.org/10.4048/jbc.2019.22.e49
Popat S, Smith IE (2005) Re: neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97:858–858. https://doi.org/10.1093/jnci/dji147
Kaufmann M, von Minckwitz G, Mamounas EP et al (2012) Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol 19:1508–1516. https://doi.org/10.1245/s10434-011-2108-2
Mamounas EP (2018) Optimizing surgical management of the axilla after neoadjuvant chemotherapy: an evolving story. Ann Surg Oncol 25:2124–2126. https://doi.org/10.1245/s10434-018-6537-z
Navarro-Cecilia J, Dueñas-Rodríguez B, Luque-López C et al (2013) Intraoperative sentinel node biopsy by one-step nucleic acid amplification (OSNA) avoids axillary lymphadenectomy in women with breast cancer treated with neoadjuvant chemotherapy. Eur J Surg Oncol (EJSO) 39:873–879. https://doi.org/10.1016/j.ejso.2013.05.002
Loibl S, von Minckwitz G, Raab G et al (2006) Surgical procedures after neoadjuvant chemotherapy in operable breast cancer: results of the GEPARDUO trial. Ann Surg Oncol 13:1434–1442. https://doi.org/10.1245/s10434-006-9011-2
Pecha V, Kolarik D, Kozevnikova R et al (2011) Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Cancer 117:4606–4616. https://doi.org/10.1002/cncr.26102
Vieites B, López-García MÁ, Castilla C et al (2016) CK19 expression in breast tumours and lymph node metastasis after neoadjuvant therapy. Histopathology 69:239–249. https://doi.org/10.1111/his.12924
Chu PG, Weiss LM (2002) Keratin expression in human tissues and neoplasms. Histopathology 40:403–439. https://doi.org/10.1046/j.1365-2559.2002.01387.x
Espinosa-Bravo M, Navarro-Cecilia J, Ramos Boyero M et al (2017) Intraoperative assessment of sentinel lymph node by one-step nucleic acid amplification in breast cancer patients after neoadjuvant treatment reduces the need for a second surgery for axillary lymph node dissection. Breast 31:40–45. https://doi.org/10.1016/j.breast.2016.10.002
Takamoto K, Shimazu K, Naoi Y et al (2016) One-step nucleic acid amplification assay for detection of axillary lymph node metastases in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 23:78–86. https://doi.org/10.1245/s10434-015-4693-y
Espinosa-Bravo M, Sansano I, Pérez-Hoyos S et al (2013) Prediction of non-sentinel lymph node metastasis in early breast cancer by assessing total tumoral load in the sentinel lymph node by molecular assay. Eur J Surg Oncol (EJSO) 39:766–773. https://doi.org/10.1016/j.ejso.2013.03.011
Peg V, Espinosa-Bravo M, Vieites B et al (2013) Intraoperative molecular analysis of total tumor load in sentinel lymph node: a new predictor of axillary status in early breast cancer patients. Breast Cancer Res Treat 139:87–93. https://doi.org/10.1007/s10549-013-2524-z
Peg V, Sansano I, Vieites B et al (2017) Role of total tumour load of sentinel lymph node on survival in early breast cancer patients. The Breast 33:8–13. https://doi.org/10.1016/j.breast.2017.02.011
Vieites B, López-García MÁ, Martín-Salvago MD et al (2021) Predictive and prognostic value of total tumor load in sentinel lymph nodes in breast cancer patients after neoadjuvant treatment using one-step nucleic acid amplification: the NEOVATTL study. Clin Transl Oncol 23:1377–1385. https://doi.org/10.1007/s12094-020-02530-4
Rebollo-Aguirre ÁC, Gallego-Peinado M, Sánchez-Sánchez R et al (2013) Biopsia del ganglio centinela después de la quimioterapia neoadyuvante en pacientes con cáncer de mama operable y ganglios axilares positivos al diagnóstico. Rev Esp Med Nucl Imagen Mol 32:240–245. https://doi.org/10.1016/j.remn.2013.03.006
Rubio IT, Espinosa-Bravo M, Rodrigo M et al (2014) Nomogram including the total tumoral load in the sentinel nodes assessed by one-step nucleic acid amplification as a new factor for predicting nonsentinel lymph node metastasis in breast cancer patients. Breast Cancer Res Treat 147:371–380. https://doi.org/10.1007/s10549-014-3108-2
Hunter-Smith AE, Rayter Z (2018) One-step nucleic acid amplification: the possible value in assessing sentinel lymph node metastasis during mastectomy. Breast Cancer 10:13–21. https://doi.org/10.2147/BCTT.S113737
Tsujimoto M, Nakabayashi K, Yoshidome K et al (2007) One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res 13:4807–4816. https://doi.org/10.1158/1078-0432.CCR-06-2512
Laohawiriyakamol S, Mahattanobon S, Puttawibul P (2022) Intraoperative molecular analysis of total tumor load in sentinel lymph node: a predictor of axillary status in early breast cancer. Asian Pacific J Cancer Prevent 23:349–354. https://doi.org/10.31557/APJCP.2022.23.1.349
Piñero A, Canteras M, Moreno A et al (2013) Multicenter validation of two nomograms to predict non-sentinel node involvement in breast cancer. Clin Transl Oncol 15:117–123. https://doi.org/10.1007/s12094-012-0887-y
di Filippo F, Giannarelli D, Bouteille C et al (2015) Elaboration of a nomogram to predict non sentinel node status in breast cancer patients with positive sentinel node, intra-operatively assessed with one step nucleic acid amplification method. J Exp Clin Cancer Res 34:136. https://doi.org/10.1186/s13046-015-0246-2
Ohi Y, Umekita Y, Sagara Y et al (2012) Whole sentinel lymph node analysis by a molecular assay predicts axillary node status in breast cancer. Br J Cancer 107:1239–1243. https://doi.org/10.1038/bjc.2012.387
Teramoto A, Shimazu K, Naoi Y et al (2014) One-step nucleic acid amplification assay for intraoperative prediction of non-sentinel lymph node metastasis in breast cancer patients with sentinel lymph node metastasis. The Breast 23:579–585. https://doi.org/10.1016/j.breast.2014.05.026
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948. https://doi.org/10.1056/NEJMra1001389
Vargo JA, Beriwal S, Ahrendt GM et al (2011) Molecular class as a predictor of locoregional and distant recurrence in the neoadjuvant setting for breast cancer. Oncology 80:341–349. https://doi.org/10.1159/000330203
Li X, Yang J, Peng L et al (2017) Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat 161:279–287. https://doi.org/10.1007/s10549-016-4059-6
Gonçalves H, Guerra MR, Duarte Cintra JR et al (2018) Survival study of triple-negative and non-triple-negative breast cancer in a Brazilian cohort. Clin Med Insights Oncol 12:117955491879056. https://doi.org/10.1177/1179554918790563
Giuliano AE, Ballman K, McCall L et al (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis. JAMA 318:918. https://doi.org/10.1001/jama.2017.11470
Acknowledgements
The authors thank the i2e3 Procomms team and specially, Sara Gutiérrez, MD and Sara Cervantes, Ph.D. for providing medical writing support during the preparation of the manuscript.
Funding
This work was supported by Sysmex in the medical writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by BV, MDM and MS. The first draft of the manuscript was written by BV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martín-Salvago, M.D., Sancho, M., López-García, M.Á. et al. Value of total tumor load as a clinical and pathological factor in the prognosis of breast cancer patients receiving neoadjuvant treatment. Comparison of three populations with three different surgical approaches: NEOVATTL Pro 3 Study. Breast Cancer Res Treat 200, 203–215 (2023). https://doi.org/10.1007/s10549-023-06954-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06954-8