Abstract
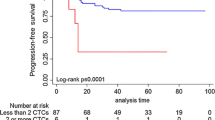
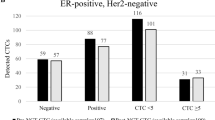
Neoadjuvant chemotherapy (NCT) is the standard treatment for patients with locally advanced breast cancer (LABC). The predictive value of heterogeneous circulating tumor cells (CTCs) in NCT response has not been determined. All patients were staged as LABC, and blood samples were collected at the time of biopsy, and after the first and eighth NCT courses. Patients were divided into High responders (High-R) and Low responders (Low-R) according to Miller–Payne system and changes in Ki-67 levels after NCT treatment. A novel SE-i·FISH strategy was applied to detect CTCs. Heterogeneities were successfully analyzed in patients undergoing NCT. Total CTCs increased continuously and were higher in Low-R group, while in High-R group, CTCs increased slightly during NCT before returning to baseline levels. Triploid and tetraploid chromosome 8 increased in Low-R but not High-R group. The number of small CTCs in Low-R group increased significantly until the last sample, however, remained constant in High-R group. The patients with more CTCs had shorter PFS and OS than those with less CTCs after the eighth course of NCT. Total CTCs following NCT could predict patients’ responses. More detailed characterizations of CTC blood profiles may improve predictive capacity and treatments of LABC.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Other remaining data and materials are available from the authors upon reasonable request.
References
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Yamauchi K et al (2008) Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res 68(2):516–520
Comen E, Norton L, Massagué J (2011) Clinical implications of cancer self-seeding. Nat Rev Clin Oncol 8(6):369–377
Bidard FC et al (2018) Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst 110(6):560–567
Caixeiro NJ et al (2014) Circulating tumour cells–a bona fide cause of metastatic cancer. Cancer Metastasis Rev 33(2–3):747–756
Molloy TJ et al (2011) The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res 13(3):R61
Chong MH et al (2012) The dynamic change of circulating tumour cells in patients with operable breast cancer before and after chemotherapy based on a multimarker QPCR platform. Br J Cancer 106(10):1605–1610
van Dalum G et al (2015) Circulating tumor cells before and during follow-up after breast cancer surgery. Int J Oncol 46(1):407–413
Nolé F et al (2008) Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol 19(5):891–897
Pierga JY et al (2017) Circulating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol 28(1):103–109
Riethdorf S et al (2017) Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant “Geparquattro” trial. Clin Cancer Res 23(18):5384–5393
Andergassen U et al (2016) Real-time RT-PCR systems for CTC detection from blood samples of breast cancer and gynaecological tumour patients (review). Oncol Rep 35(4):1905–1915
Ozkumur E et al (2013) Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med 5(179):17947
Passerini V et al (2016) The presence of extra chromosomes leads to genomic instability. Nat Commun 7:10754
Gordon DJ, Resio B, Pellman D (2012) Causes and consequences of aneuploidy in cancer. Nat Rev Genet 13(3):189–203
Kops GJ, Weaver BA, Cleveland DW (2005) On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5(10):773–785
Ogston KN et al (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12(5):320–327
Lin PP (2015) Integrated EpCAM-independent subtraction enrichment and iFISH strategies to detect and classify disseminated and circulating tumors cells. Clin Transl Med 4(1):38
Lin PP et al (2017) Comprehensive in situ co-detection of aneuploid circulating endothelial and tumor cells. Sci Rep 7(1):9789
Magee R, Rigoutsos I (2020) On the expanding roles of tRNA fragments in modulating cell behavior. Nucleic Acids Res 48(17):9433–9448
Heitzer E et al (2019) Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 20(2):71–88
Matikas A et al (2022) Detection of circulating tumour cells before and following adjuvant chemotherapy and long-term prognosis of early breast cancer. Br J Cancer 126(11):1563–1569
Pierga JY et al (2008) Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res 14(21):7004–7010
Onstenk W et al (2015) Improved circulating tumor cell detection by a combined EpCAM and MCAM cell search enrichment approach in patients with breast cancer undergoing neoadjuvant chemotherapy. Mol Cancer Ther 14(3):821–827
Kasimir-Bauer S et al (2016) Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res 18(1):20
Mego M et al (2012) Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: The effect of neoadjuvant therapy. Int J Cancer 130(4):808–816
Li X et al (2018) Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget 9(2):2705–2714
Zhang J et al (2018) Circulating tumor cells with karyotyping as a novel biomarker for diagnosis and treatment of nasopharyngeal carcinoma. BMC Cancer 18(1):1133
Ye Z et al (2019) Detecting and phenotyping of aneuploid circulating tumor cells in patients with various malignancies. Cancer Biol Ther 20(4):546–551
Luo S et al (2021) Optimal strategy for colorectal cancer patients’ diagnosis based on circulating tumor cells and circulating tumor endothelial cells by subtraction enrichment and immunostaining-fluorescence in situ hybridization combining with CEA and CA19-9. J Oncol 2021:1517488
Lin AY et al (2021) Identification and comprehensive co-detection of necrotic and viable aneuploid cancer cells in peripheral blood. Cancers (Basel) 13(20):5108
Zhang T et al (2021) Role of aneuploid circulating tumor cells and CD31(+) circulating tumor endothelial cells in predicting and monitoring anti-angiogenic therapy efficacy in advanced NSCLC. Mol Oncol 15(11):2891–2909
Mi J et al (2022) Case report: post-therapeutic laryngeal carcinoma patient possessing a high ratio of aneuploid CTECs to CTCs rapidly developed de novo malignancy in pancreas. Front Oncol 12:981907
Camara O et al (2007) The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann Oncol 18(9):1484–1492
Ignatiadis M et al (2008) Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res 14(9):2593–2600
Pachmann K et al (2008) Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol 26(8):1208–1215
Fisher B, Fisher ER (1967) The organ distribution of disseminated 51 Cr-labeled tumor cells. Cancer Res 27(2):412–420
Fisher B, Fisher ER, Feduska N (1967) Trauma and the localization of tumor cells. Cancer 20(1):23–30
Miglietta L et al (2010) Prognostic value of estrogen receptor and Ki-67 index after neoadjuvant chemotherapy in locally advanced breast cancer expressing high levels of proliferation at diagnosis. Oncology 79(3–4):255–261
Li L et al (2017) Prognostic values of Ki-67 in neoadjuvant setting for breast cancer: a systematic review and meta-analysis. Future Oncol 13(11):1021–1034
Miglietta L et al (2013) A prognostic model based on combining estrogen receptor expression and Ki-67 value after neoadjuvant chemotherapy predicts clinical outcome in locally advanced breast cancer: extension and analysis of a previously reported cohort of patients. Eur J Surg Oncol 39(10):1046–1052
Lee AJ et al (2011) Chromosomal instability confers intrinsic multidrug resistance. Cancer Res 71(5):1858–1870
Li Y et al (2014) Clinical significance of phenotyping and karyotyping of circulating tumor cells in patients with advanced gastric cancer. Oncotarget 5(16):6594–6602
Wan JF et al (2018) Aneuploidy of chromosome 8 and mutation of circulating tumor cells predict pathologic complete response in the treatment of locally advanced rectal cancer. Oncol Lett 16(2):1863–1868
Li Y et al (2018) Evolutionary expression of HER2 conferred by chromosome aneuploidy on circulating gastric cancer cells contributes to developing targeted and chemotherapeutic resistance. Clin Cancer Res 24(21):5261–5271
Ito H et al (2016) Change in number and size of circulating tumor cells with high telomerase activity during treatment of patients with gastric cancer. Oncol Lett 12(6):4720–4726
Aceto N et al (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158(5):1110–1122
Zhang D et al (2017) Circulating tumor microemboli (CTM) and vimentin+ circulating tumor cells (CTCs) detected by a size-based platform predict worse prognosis in advanced colorectal cancer patients during chemotherapy. Cancer Cell Int 17:6
Li QQ et al (2011) Involvement of NF-kappaB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ 18(1):16–25
Chockley PJ et al (2018) Epithelial-mesenchymal transition leads to NK cell-mediated metastasis-specific immunosurveillance in lung cancer. J Clin Invest 128(4):1384–1396
Somasundaram R, Herlyn D (2009) Chemokines and the microenvironment in neuroectodermal tumor-host interaction. Semin Cancer Biol 19(2):92–96
Coupland LA, Chong BH, Parish CR (2012) Platelets and P-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res 72(18):4662–4671
Reymond N, d’Agua BB, Ridley AJ (2013) Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13(12):858–870
Smerage JB et al (2014) Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 32(31):3483–3489
Kim YR et al (2018) Selective killing of circulating tumor cells prevents metastasis and extends survival. J Hematol Oncol 11(1):114
Acknowledgements
The authors wish to thank all the patients and family members for participating in this study.
Funding
This work was supported by the Natural Science Foundation of China (81572607, 82203118 and 81572602).
Author information
Authors and Affiliations
Contributions
GM carried out the conceptualization, project administration, formal analysis, writing – original draft, and writing – review and editing. JW carried out project administration and data analysis. JF carried out the data curation and methodology. RC participated in project administration and data processing. ML participated in writing – original draft and writing – review and editing. ML carried out the data analysis. TX carried out the conceptualization and project administration. XL carried out the conceptualization and funding acquisition. SW carried out the conceptualization, supervision, and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest. Prior presentation: This work has been presented in part at the 2019 ASCO Annual Meeting.
Ethical approval
Samples were collected at the First Affiliated Hospital with Nanjing Medical University. Ethics approval was obtained from the institutional ethics committee of the First Affiliated Hospital with Nanjing Medical University.
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, G., Wang, J., Fu, J. et al. Heterogeneous circulating tumor cells correlate with responses to neoadjuvant chemotherapy and prognosis in patients with locally advanced breast cancer. Breast Cancer Res Treat 201, 27–41 (2023). https://doi.org/10.1007/s10549-023-06942-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06942-y