Abstract
Background
Triple-negative breast cancer (TNBC) is characterized by a lack of estrogen and progesterone receptor expression and HER-2 gene amplification. Circulating tumor cells (CTCs) can be identified in 25 % of nonmetastatic breast cancer patients, and the identification of ≥1 CTC predicts outcome. This study was designed to determine whether CTCs present after neoadjuvant chemotherapy (NACT) predicted worse outcome in nonmetastatic TNBC patients.
Methods
CTCs were assessed in 57 TNBC patients with nonmetastatic TNBC after the completion of NACT. CTCs (per 7.5 ml blood) were identified using the Cell Search® System (Janssen). Log-rank test and Cox regression analysis were applied to establish the association of CTCs with relapse-free (RFS) and overall survival (OS).
Results
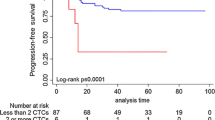
Median follow-up was 30 months, and mean age was 53 years. Fifty-four patients (95 %) had >2-cm tumors, 42 (84 %) were nuclear grade 3, and 42 (74 %) had positive axillary lymph nodes. One or more CTC was identified in 30 % of patients. CTC presence was not associated with primary tumor size, high grade, or lymph node positivity. Multivariate analysis demonstrated that detection of ≥1 CTC predicted decreased RFS (log-rank P = 0.03, HR 5.25, 95 % CI 1.34–20.56) and OS (log-rank P = 0.03, HR 7.04, 95 % CI 1.26–39.35).
Conclusions
One or more CTCs present after NACT predicted relapse and survival in nonmetastatic TNBC patients. This information would be helpful in future clinical trial design of adjuvant treatments for TNBC patients who are at risk for relapse after completing NACT.

Similar content being viewed by others
References
Diaz LK, Cryns VL, Symmans WF, Sneige N. Triple negative breast carcinoma and the basal phenotype: from expression profiling to clinical practice. Adv Anat Pathol. 2007;14(6):419–30.
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81.
Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34.
Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20(4):621–7.
Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7.
Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27(8):1168–76.
Kaufmann M, von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–34.
Sachelarie I, Grossbard ML, Chadha M, Feldman S, Ghesani M, Blum RH. Primary systemic therapy of breast cancer. Oncologist. 2006;11(6):574–89.
Bonnefoi H, Litiere S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014;25(6):1128–36.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9.
von Minckwitz G. Neoadjuvant chemotherapy in breast cancer-insights from the German experience. Breast cancer. 2012;19(4):282–8.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
Smith BL. Neoadjuvant versus adjuvant systemic therapy for operable breast cancer: equivalent outcomes? Ann Surg. 2013;257(2):180–1.
Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6(6):339–51.
Naume B, Borgen E, Beiske K, et al. Immunomagnetic techniques for the enrichment and detection of isolated breast carcinoma cells in bone marrow and peripheral blood. J Hematother. 1997;6(2):103–14.
Bidard FC, Mathiot C, Delaloge S, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21(4):729–33.
Rack B, Schindlbeck C, Juckstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106(5).
Franken B, de Groot MR, Mastboom WJ, et al. Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res. 2012;14(5):R133.
van Dalum G, van der Stam GJ, Tibbe AG, et al. Circulating tumor cells before and during follow-up after breast cancer surgery. Int J Oncol. 2015;46(1):407–13.
Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–95.
Edge SB. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105(1):97–102.
Krishnamurthy S, Cristofanilli M, Singh B, et al. Detection of minimal residual disease in blood and bone marrow in early stage breast cancer. Cancer. 2010;116(14):3330–7.
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91.
McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur J Cancer. 2005;41(12):1690–6.
Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–32.
Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14(21):7004–10.
Wulfing P, Borchard J, Buerger H, et al. HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res. 2006;12(6):1715–20.
Karhade M, Hall C, Mishra P, et al. Circulating tumor cells in non-metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2014;147(2):325–33.
Muller V, Stahmann N, Riethdorf S, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11(10):3678–85.
Gazzaniga P, Naso G, Gradilone A, et al. Chemosensitivity profile assay of circulating cancer cells: prognostic and predictive value in epithelial tumors. Int J Cancer. 2010;126(10):2437–47.
Gradilone A, Naso G, Raimondi C, et al. Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance and phenotypic characterization. Ann Oncol. 2011;22(1):86–92.
Apostolaki S, Perraki M, Pallis A, et al. Circulating HER2 mRNA-positive cells in the peripheral blood of patients with stage I and II breast cancer after the administration of adjuvant chemotherapy: evaluation of their clinical relevance. Ann Oncol. 2007;18(5):851–8.
Fehm T, Muller V, Aktas B, et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat. 2010;124(2):403–12.
Ignatiadis M, Rothe F, Chaboteaux C, et al. HER2-positive circulating tumor cells in breast cancer. PloS One. 2011;6(1):e15624.
Meng S, Tripathy D, Shete S, et al. HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A. 2004;101(25):9393–8.
Riethdorf S, Muller V, Zhang L, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res. 2010;16(9):2634–45.
Tewes M, Aktas B, Welt A, et al. Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat. 2009;115(3):581–90.
Nadal R, Fernandez A, Sanchez-Rovira P, et al. Biomarkers characterization of circulating tumour cells in breast cancer patients. Breast Cancer Res. 2012;14(3):R71.
Acknowledgment
This work was supported by The Society of Surgical Oncology Clinical Investigator Award (A Lucci), The Morgan Welch Inflammatory Breast Cancer Program, and The Institute for Personalized Therapy at U.T. M.D. Anderson Cancer Center, the State of Texas Rare and Aggressive Breast Cancer Research Program, and philanthropic funds for which we thank our many generous donors.
Disclosures
Dr. Lucci served as a consultant at Janssen Diagnostics in December, 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hall, C., Karhade, M., Laubacher, B. et al. Circulating Tumor Cells After Neoadjuvant Chemotherapy in Stage I–III Triple-Negative Breast Cancer. Ann Surg Oncol 22 (Suppl 3), 552–558 (2015). https://doi.org/10.1245/s10434-015-4600-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4600-6