Abstract
Background
Population mammographic screening for breast cancer has led to large increases in the diagnosis and treatment of ductal carcinoma in situ (DCIS). Active surveillance has been proposed as a management strategy for low-risk DCIS to mitigate against potential overdiagnosis and overtreatment. However, clinicians and patients remain reluctant to choose active surveillance, even within a trial setting. Re-calibration of the diagnostic threshold for low-risk DCIS and/or use of a label that does not include the word ‘cancer’ might encourage the uptake of active surveillance and other conservative treatment options. We aimed to identify and collate relevant epidemiological evidence to inform further discussion on these ideas.
Methods
We searched PubMed and EMBASE databases for low-risk DCIS studies in four categories: (1) natural history; (2) subclinical cancer found at autopsy; (3) diagnostic reproducibility (two or more pathologist interpretations at a single time point); and (4) diagnostic drift (two or more pathologist interpretations at different time points). Where we identified a pre-existing systematic review, the search was restricted to studies published after the inclusion period of the review. Two authors screened records, extracted data, and performed risk of bias assessment. We undertook a narrative synthesis of the included evidence within each category.
Results
Natural History (n = 11): one systematic review and nine primary studies were included, but only five provided evidence on the prognosis of women with low-risk DCIS. These studies reported that women with low-risk DCIS had comparable outcomes whether or not they had surgery. The risk of invasive breast cancer in patients with low-risk DCIS ranged from 6.5% (7.5 years) to 10.8% (10 years). The risk of dying from breast cancer in patients with low-risk DCIS ranged from 1.2 to 2.2% (10 years). Subclinical cancer at autopsy (n = 1): one systematic review of 13 studies estimated the mean prevalence of subclinical in situ breast cancer to be 8.9%. Diagnostic reproducibility (n = 13): two systematic reviews and 11 primary studies found at most moderate agreement in differentiating low-grade DCIS from other diagnoses. Diagnostic drift: no studies found.
Conclusion
Epidemiological evidence supports consideration of relabelling and/or recalibrating diagnostic thresholds for low-risk DCIS. Such diagnostic changes would need agreement on the definition of low-risk DCIS and improved diagnostic reproducibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Widespread use of mammographic screening for breast cancer has dramatically increased the detection of early-stage breast cancers and precursor lesions, including ductal carcinoma in situ (DCIS). As some of these lesions are unlikely to progress to clinically significant disease during the person’s lifetime if left undetected and untreated, an important harm from mammography screening programmes is overdiagnosis and subsequent overtreatment [1,2,3,4]. Epidemiological trends in the United States (US) demonstrate increasing rates of breast cancer diagnoses alongside largely stable rates of metastatic disease and breast cancer mortality [5], observations consistent with a picture of overdiagnosis.
Those diagnosed with DCIS are usually offered surgery in the form of breast-conserving surgery or mastectomy. They may also undergo axillary lymph node intervention (sentinel lymph node biopsy and sometimes axillary lymph node clearance), radiotherapy, and endocrine therapies [6]. The risks of adverse outcomes and harms from at least some of these treatments may be acceptable trade-offs against the potential life-extending benefits for high-risk lesions [3]. However, in the case of low-risk DCIS, the trade-offs may no longer be acceptable, as there is much less potential for benefit. To prevent the potential for harm from overtreatment of low-risk lesions, active surveillance has been proposed as an alternative management strategy [7, 8]. There are several ongoing international clinical trials assessing the effects of active surveillance for low-risk DCIS compared to immediate treatment (LORETTA, LORD, LORIS, COMET) [1,2,3, 9, 10]. As there is not yet a consensus on what exactly constitutes low-risk DCIS, the trials have differing criteria, based on nuclear grade, patient age, and various clinical and pathological features. If evidence from these trials show that active surveillance is a safe and effective management option, then it may be offered in routine clinical practice, similar to what is now accepted practice for low-risk prostate cancer [11]. Recruitment into the trials has been slow, likely due to clinician and patient concerns about active surveillance as a management option [12,13,14]. Further, outside of trials currently only 3% of women diagnosed with DCIS in the US choose to forego both surgery and radiotherapy [3, 4]. To encourage uptake of active surveillance for low-risk DCIS where appropriate, consideration may be given to using alternative labels to describe such lesions that do not include the word ‘cancer’ [15, 16]. Alternatively, the DCIS label could be retained, but with a recalibration of diagnostic thresholds such that the DCIS term is only applied to lesions with a higher risk of adverse outcomes [17], and low-risk DCIS is given another label that does not contain the word ‘cancer’. These possibilities may encourage both clinicians and patients to choose active surveillance and other conservative management options where clinically appropriate [18,19,20,21,22].
The purpose of this review was to systematically appraise evidence that might support or refute the case for adopting alternative non-cancer labels and/or recalibration of diagnostic thresholds, for low-risk DCIS. We sought to understand (i) the natural history of low-risk DCIS if left untreated, (ii) the size of the “reservoir” of subclinical DCIS in people who died of other causes, (iii) the diagnostic reproducibility of DCIS, and (iv) whether there has been diagnostic drift that has expanded the DCIS definition over time, for example, where the same type of lesion would be diagnosed as cancer in recent years but would not have been in the past.
Materials and methods
We searched for studies in four different categories: (1) natural history studies, including active surveillance and watchful waiting studies, (2) autopsy studies, (3) diagnostic reproducibility studies, and (4) diagnostic drift studies. PubMed and EMBASE databases were searched from inception to 8 October 2021. The search strategy is provided in Online Appendix 1. In addition, specific studies suggested by experts were also screened for potential inclusion, if they were determined to fit the inclusion criteria.
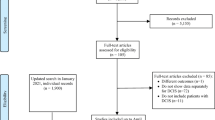
We identified an existing review for each of the natural history, autopsy, and reproducibility categories suggested by content experts, and a further review on reproducibility through initial literature searches. We therefore searched for additional studies published after the reviews’ inclusion period for these three categories. (See Online Appendix 2 for flow diagram.)
Inclusion criteria
As the definition of what constitutes “low-risk” varies, we took a broad approach to inclusion and included all evidence relevant to DCIS without restriction on risk classification. As this study was focused on DCIS, we did not specifically include evidence about other types of borderline lesions, such as atypical ductal hyperplasia (ADH) or lobular carcinoma in situ (LCIS). In the natural history category, we included prospective or retrospective cohort studies where patients were diagnosed with DCIS but did not have local treatment (surgery or radiotherapy). In the autopsy category, we included autopsy studies reporting incidental (subclinical) breast cancer in individuals with no known history of breast cancer. In the diagnostic reproducibility category, we included cross-sectional studies with two or more independent diagnostic classifications of the same histopathological slides. In the diagnostic drift category, we included longitudinal studies with two or more independent diagnostic classifications of the same histopathological slides.
Exclusion criteria
Abstracts, reviews, protocols of planned studies, and studies that were not in English were excluded. Studies where patients had only metastatic disease were also excluded. In the natural history category, studies which did not report on clinical outcomes relevant to disease progression (e.g. studies that only reported psychological outcomes) were excluded. In the autopsy category, studies only reporting cases of patients who were determined to have died of breast cancer that was not detected during life were excluded. In the diagnostic reproducibility and diagnostic drift categories, studies that reported only readings by non-pathologists, and studies that did not report on diagnostic classification, were excluded.
Study selection, data extraction, quality assessment, and synthesis
The titles and abstracts were screened by one of the authors (CRS) and full text independently screened by two authors (TM and CRS). Discrepancies were resolved through discussion or through adjudication by two other authors (BN and KJLB). One of the authors (TM) extracted and summarized relevant study data in a spreadsheet that was developed and piloted by two other authors (BN and KJLB) and then assessed studies for risk of bias. Risk of bias was assessed using standardized tools adapted from ROBINS-I [23] for natural history studies, Hoy and colleagues’ tool [24] for autopsy studies, and QUADAS-2 [25] and QAREL [26] tools for diagnostic reproducibility and diagnostic drift studies. Both data extraction and quality assessment were checked by another author (BN or KJLB). Finally, a narrative synthesis of the evidence in each category was undertaken.
Results
Natural history studies (n = 11) (details in Table 1)
One review was pre-identified in the natural history category and included. We screened titles and abstracts of 167 articles retrieved from the database search and two suggested by experts, reviewed the full text of 42 articles, and included 9 primary studies. The screening process is shown in Online Appendix 2a. Six of the primary studies were population database studies. Three studies were based on clinical records, and at least some patients received endocrine therapy in all these studies. All primary studies described in this section were retrospective studies and were assessed as having a high risk of bias overall.
The review by Erbas et al. [27] identified four studies that retrospectively examined cases of breast biopsies originally diagnosed as benign, to find cases of DCIS misdiagnosed as benign, and where follow-up data were available. Amongst the four studies, with a total of 136 cases (range 13–80 cases), the rate of development of invasive cancer (ipsilateral or contralateral) was 14–53%, after 1–31-year follow-up. No data were provided on low-risk DCIS specifically. No risk of bias assessments were reported by the reviewers.
Six population-based studies reported on evidence on DCIS outcomes from administrative databases. Four US studies utilized data from the National Cancer Institute’s surveillance, epidemiology, and end results (SEER) programme. Byng et al. [28] reported a propensity score-based analysis for 1,650 patients with DCIS managed without surgery or radiotherapy (364 grade I, 786 grade II, and 500 grade III) amongst a total 85,982 patients with DCIS. They found that patients with low-risk DCIS had a similar risk of invasive breast cancer at long-term follow-up whether they received treatment or not (low risk defined as Hispanic or non-Hispanic white ethnicity, age 50–69 at diagnosis, oestrogen receptor positive, Grade I or II, lesion size < 2 cm). The risk of ipsilateral invasive breast cancer in the untreated low-risk DCIS group was estimated to be 0.92% (95% CI 0.00–1.95%) at 5 years and 3.02% (95% CI 0.00–6.05%) at 10 years. The combined risk of contralateral and ipsilateral invasive cancer for women with untreated low-risk DCIS was within the 10-year population-wide age-specific risk of invasive breast cancer for US women. In another propensity score-based analysis, Akushevich et al. [29] compared outcomes for women aged 65 + diagnosed with DCIS who (i) had treatment within a year of diagnosis before any evidence of cancer progression (n = 21,772); (ii) had treatment within a year of diagnosis after evidence of cancer progression (n = 431); and (iii) did not have treatment within a year of diagnosis (n = 405). They found that patients who did not have treatment within a year of diagnosis (group iii) had a higher risk of all-cause (HR 3.54; 95% CI 3.29–3.82) and breast cancer-specific (HR 10.73; 95% CI 8.63–13.35) mortality compared with those who received treatment within a year of diagnosis (either before or after evidence of cancer progression). However, results for low-risk DCIS were not reported. Ryser et al. [30] found that amongst 1286 DCIS patients who did not have local treatment, 111 patients (8.6%) were diagnosed with ipsilateral invasive cancer after a median 5.5 years of follow-up. Amongst the 239 patients with low-risk DCIS (non-high grade and ER or PR positive diagnosed in women 40 years or older), the cumulative net risk of ipsilateral invasive cancer was 6.5% (95% CI 3.5–12.0%) after 7.5-year follow-up. Sagara et al. [31] reported outcomes on 1169 patients with DCIS managed without surgery amongst a total of 57,222 patients with DCIS. They found that the degree of survival benefit conferred by surgery differed by nuclear grade (p = 0.003), after adjusting for other relevant patient characteristics. For low nuclear-grade DCIS, there was no significant difference in the weighted 10-year breast cancer-specific survival between the surgery and non-surgery groups (98.6% and 98.8%, respectively, p = 0.95).
The UK study by Mannu et al. [32] reported outcomes for 1452 patients with DCIS managed without surgery amongst a total of 35,024 patients with DCIS. They found that compared with women in the general population, women with screen-detected DCIS (whether they had surgery or not) had a higher long-term risk of developing invasive breast cancer (ipsilateral or contralateral), for at least 20 years after diagnosis. At 20 years after diagnosis, the cumulative risk of invasive breast cancer was 15.6%, and the cumulative risk of death from breast cancer was 3.8%. The authors also found that women with DCIS who did not have surgery had higher rates of invasive cancer and breast cancer mortality, compared with those who had surgery. The observed to expected ratio for death caused by breast cancer was 1.72 (1.38 to 2.12) for women who had received surgery and 3.89 (2.31 to 6.57) for women who had not received surgery. However, results for low-risk DCIS were not reported. The Hong Kong study by Co et al. [33] reported on 280 patients with DCIS managed without surgery amongst a total 1391 patients, utilizing the local cancer registry. They found that 10-year breast cancer-specific survival was 97.8% for low-risk DCIS without surgery (patients aged 46–70 years with low or intermediate nuclear-grade DCIS). The incidence of subsequent invasive cancer was similar for patients with low-risk DCIS for those who had surgery (989.4 per 100,000) and those who did not (716 per 100 000 person years; p = 0.64).
Three retrospective cohort studies reported on evidence on DCIS outcomes from clinic databases. The UK study by Maxwell et al. [34] reported on 89 patients with DCIS, including 17 with low-grade DCIS, who did not have surgical treatment as identified via cancer registries, NHS programmes, and clinical studies. Overall, 29 cases (33%) were diagnosed with invasive cancer, after a median of 3 years and 9 months follow-up. Amongst 17 patients with low-grade DCIS, 3 (18%) were diagnosed with invasive cancer, after a median of 4 years and 3 months. The US study by Grimm et al. [35] reported on follow-up of 29 patients, including one case of low-grade DCIS who did not develop invasive cancer. The US study by Meyerson et al. [36] reported on 14 patients with DCIS, including 4 with low-grade DCIS. Five patients, including one with low-grade DCIS, were diagnosed with invasive cancer, whilst the other 9 remained free of invasive cancer after a median follow-up of 2 years and 7.8 months.
The details of individual studies included in this section are listed in Table 1. All the primary studies identified in this category were assessed to be at high risk of bias overall. The risk of bias assessment for each study is available in Appendix 3a.
Autopsy studies (n = 1)
For autopsy studies we pre-identified a systematic review by Thomas et al. [37]. We retrieved 73 articles from the literature search, published after 8 April 2016 (the cut-off date for the Thomas review). After title and abstract screening, 5 articles were retained for full-text screening, but none of these fulfilled the inclusion criteria. Therefore, only the evidence from the pre-identified systematic review was included. The screening process is shown in Online Appendix 2b.
The systematic review included 13 studies providing 14 datasets, published from 1954 to 2015. It included a total of 2363 autopsies with 99 cases of incidental subclinical breast cancer or precursor lesions, from Denmark, Italy, Canada, the USA, Chile, Japan, Ghana, Australia, and Norway. The mean prevalence of ‘in situ cancer’, including DCIS and lobular carcinoma in situ (LCIS), was 4.5% (range 0–18.7%), whilst the mean prevalence of invasive cancer was 1.5% (range 0–7.1%). However, studies which conducted more thorough pathology examinations yielded greater prevalence of subclinical in situ cancer. After modelled adjustment for the less thorough studies, the mean prevalence of subclinical in situ cancer was estimated to be 8.9%. There was no statistically significant trend in the overall prevalence of breast cancer and its precursor lesions over time, nor with age.
Diagnostic reproducibility studies (n = 13) (details in Table 2)
Two reviews and one primary study suggested by experts were included in the reproducibility category. We screened titles and abstracts of 221 articles, reviewed the full text of 57, and included a further 10 primary studies. Therefore, we reviewed evidence from 2 reviews and 11 primary studies. The screening process is shown in Online Appendix 2c.
The review by Segnan et al. [38], which focused on the reproducibility of the diagnosis of breast lesions as benign, DCIS, or invasive cancer, included 27 studies of diagnostic reproducibility, with a total of 13,017 lesions. It found that there was a high level of heterogeneity between studies, and overall there was a non-negligible false-positive rate. Amongst 6 studies with consecutive, random, or stratified samples, 0 to 3.2% of benign lesions were misclassified as DCIS. Amongst 5 studies with only cases selected for a second opinion, 0 to 34.48% of benign lesions were misclassified as DCIS. Amongst five studies with enriched samples, 0 to 9.64% of benign lesions were misclassified as DCIS. Of the included studies, the US study by Elmore et al. [39] is notable for having both a large number of lesions examined (n = 240) and a large number of readers (n = 115 + 3). In this study, there was 75.3% (95% CI 73.4–77.0%) overall agreement amongst the readers regarding the diagnosis of breast lesions as benign without atypia, atypia, DCIS, or invasive cancer. However, benign lesions without atypia were misclassified as DCIS 2.22% of the time and lesions with atypia were misclassified as DCIS 17.1% of the time [39].
The review by Van Bockstal et al. [40], which focused on the reproducibility of the grading of DCIS lesions, included 12 studies of diagnostic reproducibility with a total of 1301 lesions. It found that all studies showed substantial interobserver variability in the assessment of parameters used in different grading systems, including nuclear grade, presence of comedo necrosis, and DCIS growth patterns. The kappa values for these parameters ranged from 0.23 (fair agreement) to 0.78 (substantial agreement), with most values < 0.60 (i.e. moderate agreement at best). The authors suggested that two-tier histopathologic assessment (i.e. classifying DCIS into two-grade categories rather than three), the implementation of digital pathology and deep learning algorithms and additional immunohistochemical and molecular testing, might be able to improve reproducibility.
In the new primary studies we identified, 8 studies reported on the differentiation between DCIS and other diagnoses. The US study by Mercan et al. [41] found that, in their sample of pathologists, the sensitivity and specificity for the differentiation of DCIS vs atypia was 0.70 and 0.82, respectively. The US study by Qiu et al. [42] found 84% agreement for diagnosis of the category ‘papilloma with atypical ductal hyperplasia or DCIS’ (k = 0.74). The US study by Brunye et al. [43] found that amongst cases with a consensus diagnosis of DCIS, there was only 52% mean concordance with the consensus, with 17% providing an ‘above consensus diagnosis’ and 31% a ‘below consensus diagnosis’. The US study by Jackson et al. [44] found 79% (95% CI 76–81%) interobserver agreement and 84% (95% CI 81–87%) intraobserver agreement for the diagnosis of DCIS. The US study by Tozbikian et al. [45], in which five pathologists who specialized in breast pathology were asked to diagnose borderline atypical ductal hyperplasia (ADH)/DCIS lesions as either benign, ADH, or DCIS, found total agreement in only 30% of cases. The German study by Trocchi et al. [46], which assessed intraobserver agreement in two experienced breast pathologists, found almost perfect agreement between the first and second assessments (chance-corrected k > 0.8 for both pathologists), when using the B-categorization scheme. The UK study by Rakha et al. [47], reporting results from the NHS Breast Screening Programme external quality assessment scheme, found a high level of reproducibility in the diagnosis of DCIS (k = 0.88). The 2016 UK study by Rakha et al. [48], reporting from the same programme, found that 28% of pathologists had misclassified cases of invasive papillary breast cancer as being in situ, whilst 11% of pathologists had misclassified a single case of papillary DCIS as benign or atypia.
Three of the new studies reported on the grading of DCIS cases. Similar to the Van Bockstal review, all three studies found only limited agreement amongst pathologists. The study by Van Seijen et al. [49], involving breast pathologists from the UK, the US, and the Netherlands, also found only moderate agreement (k = 0.50) on DCIS grading. It also found sub-optimal diagnostic reproducibility of various histological features, including necrosis, calcifications, lymphocytic infiltrate, periductal fibrosis, mitoses, and architectural pattern (k = 0.33 to 0.61). The Japanese study by Tsuda et al. [50] found that interobserver agreement of nuclear grade was moderate (k < 0.6). However, interobserver agreement of comedo necrosis was substantial (k = 0.753). The US study by Onega et al. [51] found that agreement with the reference standard diagnosis was 46% (95% CI 42–51%) for low-grade DCIS and 83% (95% CI 81–86%) for high-grade DCIS. Low-grade DCIS cases were diagnosed as high-grade or invasive cancer 23% of the time and diagnosed as benign or atypia 30% of the time.
The details of individual studies included in this section are listed in Table 2. All primary studies in this section were rated as having either low or moderate risk of bias. The risk of bias assessment for each study is available in Appendix 3b.
Diagnostic drift studies (n = 0)
No relevant reviews were identified for this category. We retrieved 254 articles from the literature search. After title and abstract screening, 39 articles were retained for full-text screening, but none of these fulfilled the inclusion criteria. The screening process is shown in Online Appendix 2d.
Discussion
This review found several lines of evidence relevant to the consideration of relabelling low-risk DCIS and/or recalibrating the diagnostic threshold of DCIS. Whilst there have been no completed prospective active surveillance trials, evidence about the natural history of DCIS is available from retrospective database reviews and retrospective cohort studies. Overall, DCIS is associated with a non-negligible risk of invasive cancer and breast cancer death if untreated; however, this appears to be lower for low-risk DCIS. It is possible that the low-risk lesions may be a risk indicator for invasive cancer rather than a direct precursor lesion. This is supported by our finding of similar outcomes on long-term follow-up of low-risk DCIS whether or not they were treated with surgery. The autopsy studies demonstrate a reservoir of subclinical in situ breast cancer that had not caused symptoms or contributed to the women’s deaths. Together, these two lines of evidence support consideration of offering active surveillance to manage low-risk DCIS. The diagnostic reproducibility evidence is mixed, and there is clearly sub-optimal agreement in the grading of DCIS. Finally, we found no evidence regarding diagnostic drift.
A major issue that needs to be resolved before relabelling low-risk DCIS and/or recalibrating the diagnostic threshold of DCIS can potentially occur is the current lack of agreement on how low-risk DCIS is to be defined. The natural history studies we found used different definitions, as do the trials that are currently underway. The diagnostic reproducibility of DCIS grading, which is part of the criteria used to classify DCIS as low risk, is also sub-optimal. If low-risk DCIS is to become a separate diagnostic category with different treatment recommendations, there will need to be an agreed upon definition for what constitutes a low-risk lesion, and the diagnostic reproducibility of such lesions must be improved.
The results of this review are consistent with the understanding that screening for breast cancer leads to overdiagnosis and overtreatment, i.e. the diagnosis and treatment of lesions that would remain clinically insignificant [16, 17, 52]. The extent overdiagnosis has been under-appreciated [15]. In particular, although DCIS was rarely diagnosed before screening, it is now routinely treated with aggressive therapy, despite uncertainty about its natural history [6], including whether some cases are already metastatic by the time they are detectable [53]. At the population level, aggressive treatment of DCIS has not led to a drop in the incidence of invasive cancer [15, 17] or metastatic cancer [53]. At the individual level, a recent RCT found that residual DCIS (left after surgery) appeared to make little difference to the effect of neoadjuvant chemotherapy on risk of cancer recurrence [54]. In recent years, active surveillance has been proposed as an alternative for low-risk DCIS. However, there remains a perception that aggressive treatment is required for cancer, and patients are often hesitant to choose active surveillance [16]. This has led to proposals for relabelling low-grade DCIS using terminology that does not include the word carcinoma, to reflect its indolent nature, and to encourage the adoption of less aggressive treatment options [15,16,17, 55]. Terms like “indolent lesions of epithelial origin” (IDLE) [17] and “ductal intraepithelial neoplasia” (DIN) [56] have been proposed. It has been shown that, in a hypothetical scenario, more women preferred surgical treatment when DCIS was described as a cancer, compared to describing it as a ‘breast lesion’ or ‘abnormal cells’ [16].
Overdiagnosis and overtreatment of DCIS are complex problems and a multi-pronged strategy will be needed to wind back the harms resulting from them. Changing the terminology used to describe low-risk lesions may form part of the overall strategy, but is unlikely to solve these problems on its own. If terminology change is thought to have merit, in order for this to be implemented there would first need to be an agreement on what is classified as low risk. Widespread mammography screening has unearthed a spectrum of subclinical breast lesions, which exist across a continuum of risk [57]. As with the detection of other asymptomatic conditions, choosing the threshold for dichotomising into low-risk versus high-risk categories is fraught. The increasing availability of molecular indicators [57,58,59,60,61] and artificial intelligence prediction of clinically aggressive behaviour [62, 63] may help with this determination. There is also the further issue that even lesions classified low risk and not labelled ‘cancer’ may be overtreated, as seen by ADH overtreatment [64, 65]. This underlies the importance of clear communication about the risk of adverse outcomes in each case, regardless of the diagnostic label used [66]. There also needs to be a more sophisticated understanding of the broad clinical spectrum of disease for breast lesions detected using modern screening technology.
The strengths of this review lie in its methodological rigour, including a comprehensive search of the literature supplemented by articles suggested by experts in the field, and a critical appraisal of included studies. We present evidence on DCIS overall, as well as low-risk DCIS where this was reported. This is also the first review of the evidence with the aim of answering the question of whether low-risk DCIS should become a separate diagnostic category with a non-cancer diagnostic label. The limitations of this review include the high risk of bias and inadequate reporting in many of the included studies, with a relative lack of data on low-risk DCIS specifically. Those studies that did report on low-risk lesions differed in the criteria they used to define this. No data from active surveillance trials are available yet, nor any other prospective cohort studies. Some of the database studies did not report the risk of developing invasive cancer after diagnosis of low-risk DCIS, which is a patient-relevant outcome. However, since this outcome is also subject to overdiagnosis through diagnostic scrutiny, it may be less reliable as a prognostic indicator than breast cancer-specific mortality. We also found no studies on diagnostic drift. Diagnostic drift refers to changes in the criteria used for diagnostic classification over time and includes both explicit definition changes, e.g. definition changes in newer editions of the WHO Blue Book, and implicit changes in the definition used by pathologists to categorize lesions, which are not formally documented. There is an important gap in the evidence base here.
Randomized trials currently underway will generate definitive evidence on the safety of active surveillance [1,2,3, 9, 10, 67], but these data will not be available for some years. Meanwhile, the evidence summarized in this review may facilitate opening discussion about the benefits and harms of removal of “cancer” from the diagnostic label of low-risk DCIS. When the evidence from the trials become available, they will further inform the debate, including a more precise definition for low-risk DCIS that has wide acceptance in clinical and pathology communities.
Data availability
Enquiries about data availability should be directed to the authors.
References
Elshof LE, Tryfonidis K, Slaets L, Van Leeuwen-Stok AE, Skinner VP, Dif N et al (2015) Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—The LORD study. Eur J Cancer 51(12):1497–1510
Grimm LJ, Ryser MD, Partridge AH, Thompson AM, Thomas JS, Wesseling J et al (2017) Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann Surg Oncol 24(12):3534–3540
Hwang ES, Hyslop T, Lynch T, Frank E, Pinto D, Basila D et al (2019) The COMET (comparison of operative versus monitoring and endocrine therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open 9(3):e026797
Ryser MD, Worni M, Turner EL, Marks JR, Durrett R, Hwang ES (2016) Outcomes of active surveillance for ductal carcinoma in situ: a computational risk analysis. J Natl Cancer Inst 108(5):dvj372
Welch HG, Kramer BS, Black WC (2019) Epidemiologic signatures in cancer. N Engl J Med 381(14):1378–1386
Omling S, Houssami N, McGeechan K, Zackrisson S, Jacklyn G, Walters D et al (2021) The management of women with ductal carcinoma in situ of the breast in Australia and New Zealand between 2007 and 2016. ANZ J Surg 91(9):1784–1791
Benson JR, Jatoi I, Toi M (2016) Treatment of low-risk ductal carcinoma in situ: is nothing better than something? Lancet Oncol 17(10):e442–e451
Fallowfield L, Francis A (2016) Overtreatment of low-grade ductal carcinoma in situ. JAMA Oncol 2(3):382–383
Rea D, Francis A, Wallis M, Thomas J, Bartlett J, Bowden S et al (2017) Confusion over differences in registration and randomization criteria for the LORIS (Low-Risk DCIS) trial. Ann Surg Oncol 24(Suppl 3):566–567
Kanbayashi C, Iwata H (2017) Current approach and future perspective for ductal carcinoma in situ of the breast. Jpn J Clin Oncol 47(8):671–677
Carroll PH, Mohler JL (2018) NCCN guidelines updates: prostate cancer and prostate cancer early detection. J Natl Compr Canc Netw 16(5S):620–623
Fallowfield L, Matthews L, Jenkins V, May S, Francis A, Rae D et al (2018) Abstract OT3–08–01: interview data from women contemplating LORIS trial entry during the feasibility study. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS17-OT3-08-01
Hersch J, Nickel B, Dixon A, Jansen J, Saunders C, Houssami N, et al., editors. Treating (or Monitoring?) Low-risk ductal carcinoma in situ (DCIS): Focus groups about women’s views. 42nd annual meeting of the society for medical decision making; 2020.
Nickel B, McCaffery K, Houssami N, Jansen J, Saunders C, Spillane A et al (2020) Views of healthcare professionals about the role of active monitoring in the management of ductal carcinoma in situ (DCIS): qualitative interview study. Breast 54:99–105
Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG et al (2014) Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol 15(6):e234–e242
Nickel B, Moynihan R, Barratt A, Brito JP, McCaffery K (2018) Renaming low risk conditions labelled as cancer. BMJ (Clin Res ed) 362:k3322
Esserman LJ, Varma M (2019) Should we rename low risk cancers? BMJ (Clin Res ed) 364:k4699
McCaffery K, Nickel B, Moynihan R, Hersch J, Teixeira-Pinto A, Irwig L et al (2015) How different terminology for ductal carcinoma in situ impacts women’s concern and treatment preferences: a randomised comparison within a national community survey. BMJ Open 5:e008094
Nickel B, Barratt A, Copp T, Moynihan R, McCaffery K (2017) Words do matter: a systematic review on how different terminology for the same condition influences management preferences. BMJ Open 7(7):e014129
Nickel B, Barratt A, Hersch J, Moynihan R, Irwig L, McCaffery K (2015) How different terminology for ductal carcinoma in situ (DCIS) impacts women’s concern and management preferences: a qualitative study. Breast 24(5):673–679
Dixon PR, Tomlinson G, Pasternak JD, Mete O, Bell CM, Sawka AM et al (2019) The role of disease label in patient perceptions and treatment decisions in the setting of low-risk malignant neoplasms. JAMA Oncol 5(6):817–823
Omer ZB, Hwang ES, Esserman LJ, Howe R, Ozanne EM (2013) Impact of ductal carcinoma in situ terminology on patient treatment preferences. JAMA Intern Med 173(19):1830–1831
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin Res ed) 355:i4919
Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C et al (2012) Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 65(9):934–939
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Lucas NP, Macaskill P, Irwig L, Bogduk N (2010) The development of a quality appraisal tool for studies of diagnostic reliability (QAREL). J Clin Epidemiol 63(8):854–861
Erbas B, Provenzano E, Armes J, Gertig D (2006) The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat 97(2):135–144
Byng D, Retèl VP, Schaapveld M, Wesseling J, van Harten WH (2021) Treating (low-risk) DCIS patients: what can we learn from real-world cancer registry evidence? Breast Cancer Res Treat 187(1):187–196
Akushevich I, Yashkin AP, Greenup RA, Hwang ES (2020) A medicare-based comparative mortality analysis of active surveillance in older women with DCIS. NPJ breast cancer 6:57
Ryser MD, Weaver DL, Zhao F, Worni M, Grimm LJ, Gulati R et al (2019) Cancer outcomes in DCIS patients without locoregional treatment. J Natl Cancer Inst 111(9):952–960
Sagara Y, Mallory MA, Wong S, Aydogan F, DeSantis S, Barry WT et al (2015) Survival benefit of breast surgery for low-grade ductal carcinoma in situ a population-based cohort study. JAMA Surg 150(8):739–745
Mannu GS, Wang Z, Broggio J, Charman J, Cheung S, Kearins O et al (2020) Invasive breast cancer and breast cancer mortality after ductal carcinoma in situ in women attending for breast screening in England, 1988–2014: population based observational cohort study. BMJ (Clin Res ed) 369:m1570
Co M, Ngan RKC, Mang OWK, Tam AHP, Wong KH, Kwong A (2021) Long-term survival outcomes of “low risk” ductal carcinoma in situ from a territory-wide cancer registry. Clin Oncol 33(1):40–45
Maxwell AJ, Dodwell DJ, Pinder SE, Wallis MG, Thompson AM, Thompson A et al (2018) Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur J Surg Oncol 44(4):429–435
Grimm LJ, Ghate SV, Hwang ES, Soo MS (2017) Imaging features of patients undergoing active surveillance for ductal carcinoma in situ. Acad Radiol 24(11):1364–1371
Meyerson AF, Lessing JN, Itakura K, Hylton NM, Wolverton DE, Joe BN et al (2011) Outcome of long term active surveillance for estrogen receptor-positive ductal carcinoma in situ. Breast 20(6):529–533
Thomas ET, Del Mar C, Glasziou P, Wright G, Barratt A, Bell KJL (2017) Prevalence of incidental breast cancer and precursor lesions in autopsy studies: a systematic review and meta-analysis. BMC Cancer 17(1):808
Segnan N, Minozzi S, Ponti A, Bellisario C, Balduzzi S, González-Lorenzo M et al (2017) Estimate of false-positive breast cancer diagnoses from accuracy studies: a systematic review. J Clin Pathol 70(4):282–294
Elmore JG, Longton GM, Carney PA, Geller BM, Onega T, Tosteson AN et al (2015) Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 313(11):1122–1132
Van Bockstal MR, Berlière M, Duhoux FP, Galant C (2020) Interobserver variability in ductal carcinoma in situ of the breast. Am J Clin Pathol 154(5):596–609
Mercan E, Mehta S, Bartlett J, Shapiro LG, Weaver DL, Elmore JG (2019) Assessment of machine learning of breast pathology structures for automated differentiation of breast cancer and high-risk proliferative lesions. JAMA Netw Open. https://doi.org/10.1001/jamanetworkopen.2019.8777
Qiu L, Mais DD, Nicolas M, Nanyes J, Kist K, Nazarullah A (2019) Diagnosis of papillary breast lesions on core needle biopsy: upgrade rates and interobserver variability. Int J Surg Pathol 2019:1066896919854543
Brunye TT, Mercan E, Weaver DL, Elmore JG (2017) Accuracy is in the eyes of the pathologist: The visual interpretive process and diagnostic accuracy with digital whole slide images. J Biomed Inform 66:171–179
Jackson SL, Frederick PD, Pepe MS, Nelson HD, Weaver DL, Allison KH et al (2017) Diagnostic reproducibility: what happens when the same pathologist interprets the same breast biopsy specimen at two points in time? Ann Surg Oncol 24(5):1234–1241
Tozbikian G, Brogi E, Vallejo CE, Giri D, Murray M, Catalano J et al (2017) Atypical ductal hyperplasia bordering on ductal carcinoma in situ. Int J Surg Pathol 25(2):100–107
Trocchi P, Holzhausen HJ, Loning T, Bocker W, Schmidt-Pokrzywniak A, Thomssen C et al (2017) Intraobserver agreement on histopathologic evaluations of core breast biopsies. Breast J 23(2):215–219
Rakha EA, Ahmed MA, Aleskandarany MA, Hodi Z, Lee AH, Pinder SE et al (2017) Diagnostic concordance of breast pathologists: lessons from the national health service breast screening programme pathology external quality assurance scheme. Histopathology 70(4):632–642
Rakha EA, Ahmed MA, Ellis IO (2016) Papillary carcinoma of the breast: diagnostic agreement and management implications. Histopathology 69(5):862–870
van Seijen M, Jóźwiak K, Pinder SE, Hall A, Krishnamurthy S, Thomas JS et al (2021) Variability in grading of ductal carcinoma in situ among an international group of pathologists. J Pathol Clin Res 7(3):233–242
Tsuda H, Yoshida M, Akiyama F, Ohi Y, Kinowaki K, Kumaki N et al (2021) Nuclear grade and comedo necrosis of ductal carcinoma in situ as histopathological eligible criteria for the Japan Clinical Oncology Group 1505 trial: an interobserver agreement study. Jpn J Clin Oncol 51(3):434–443
Onega T, Weaver DL, Frederick PD, Allison KH, Tosteson ANA, Carney PA et al (2017) The diagnostic challenge of low-grade ductal carcinoma in situ. Euro J Cancer 80:39–47
Esserman LJ, Thompson IM Jr, Reid B (2013) Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA 310(8):797–798
Welch HG, Gorski DH, Albertsen PC (2016) Trends in metastatic breast and prostate cancer. N Engl J Med 374(6):596
Osdoit M, Yau C, Symmans WF, Boughey JC, Ewing CA, Balassanian R et al (2022) Association of residual ductal carcinoma in situ with breast cancer recurrence in the neoadjuvant I-SPY2 Trial. JAMA Surg. https://doi.org/10.1001/jamasurg.2022.4118
Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S et al (2010) National institutes of health state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ september 22–24, 2009. J Natl Cancer Inst 102(3):161–169
Galimberti V, Monti S, Mastropasqua MG (2013) DCIS and LCIS are confusing and outdated terms. They should be abandoned in favor of ductal intraepithelial neoplasia (DIN) and lobular intraepithelial neoplasia (LIN). Breast 22(4):431–435
Wilson GM, Dinh P, Pathmanathan N, Graham JD (2022) Ductal carcinoma in situ: molecular changes accompanying disease progression. J Mammary Gland Biol Neoplasia 27(1):101–131
McCart Reed AE, Kalita-De Croft P, Kutasovic JR, Saunus JM, Lakhani SR (2019) Recent advances in breast cancer research impacting clinical diagnostic practice. J Pathol 247(5):552–562
Pang JB, Savas P, Fellowes AP, Mir Arnau G, Kader T, Vedururu R et al (2017) Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Modern Pathol 30(7):952–963
Rebbeck CA, Xian J, Bornelöv S, Geradts J, Hobeika A, Geiger H et al (2022) Gene expression signatures of individual ductal carcinoma in situ lesions identify processes and biomarkers associated with progression towards invasive ductal carcinoma. Nat Commun 13(1):3399
Soon PS, Provan PJ, Kim E, Pathmanathan N, Graham D, Clarke CL et al (2018) Profiling differential microRNA expression between in situ, infiltrative and lympho-vascular space invasive breast cancer: a pilot study. Clin Exp Metas 35(1–2):3–13
Capurro D, Coghlan S, Pires DEV (2022) Preventing digital overdiagnosis. JAMA 327(6):525–526
Bifulco C, Piening B, Bower T, Robicsek A, Weerasinghe R, Lee S et al (2021) Identifying high-risk breast cancer using digital pathology images. Science 2021:1
Nicosia L, Latronico A, Addante F, De Santis R, Bozzini AC, Montesano M et al (2021) Atypical ductal hyperplasia after vacuum-assisted breast biopsy: can we reduce the upgrade to breast cancer to an acceptable rate? Diagnostics (Basel, Switzerland). 11(6):1120
Schiaffino S, Cozzi A, Sardanelli F (2020) An update on the management of breast atypical ductal hyperplasia. Br J Radiol 93(1110):20200117
Zadro JR, O’Keeffe M, Ferreira GE, Traeger AC, Gamble AR, Page R et al (2022) Diagnostic labels and advice for rotator cuff disease influence perceived need for shoulder surgery: an online randomised experiment. J Physiother 68(4):269–276
DCIS Precision (2022) Clinical trials—DCIS precision 2022. https://www.dcisprecision.org/clinical-trials/. Accessed 27 Oct 2022
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by a National Health and Medical Research Council (NHMRC) Investigator Grant (#1174523). Brooke Nickel is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (#1194108). Katy Bell is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (#1174523).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, T., Semsarian, C.R., Barratt, A. et al. Should low-risk DCIS lose the cancer label? An evidence review. Breast Cancer Res Treat 199, 415–433 (2023). https://doi.org/10.1007/s10549-023-06934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06934-y