Abstract
Purpose
This study aimed to investigate the association between serum cholesterol and triglyceride levels and breast cancer risk in Japanese women.
Methods
We retrospectively evaluated the association between the levels of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TGs) and the incidence of breast cancer in a cohort study by using the health insurance claims and health checkup data from a database provided by JMDC Inc. We included 956,390 women who were insured between April 2008 and June 2019, identified breast cancer cases by using validated definitions, and estimated the risk of breast cancer by using multivariable Cox proportional hazards regression models adjusted for potential confounders.
Results
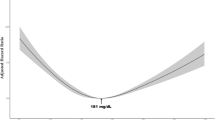
During the 2,832,277 person-years observation period (median 2.4 years), 6284 participants were diagnosed with breast cancer. There was marginally significant association between LDL-C and breast cancer risk when comparing the highest and lowest quintiles and at the clinical cutoff values for diagnosing hyperlipidemia. HDL-C was not associated with breast cancer. However, when stratified by age groups (< 50 and ≥ 50), HDL-C was inversely associated with breast cancer risk in women over 50 years old. TG was not associated with breast cancer risk.
Conclusion
In this population, there was a modest association of LDL-C at the clinical cutoff values for diagnosing hyperlipidemia (140 mg/mL), and there were no associations of HDL-C and TG with breast cancer risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignant disease worldwide, accounting for 24% of new cancer cases and 15% of cancer-related deaths in 2018 [1]. The incidence of breast cancer is also increasing in Japan. In 2018, it was the most common malignancy among women, with more than 90,000 new cases.
However, the role of cholesterol in cancer development remains controversial [2], and the association between cholesterol levels and breast cancer has not yielded consistent results. Comprehensive meta-analysis studies found an inverse association between high-density lipoprotein cholesterol (HDL-C) and breast cancer risk in postmenopausal or premenopausal women, but there was no association between low-density lipoprotein cholesterol (LDL-C) and breast cancer risk [3, 4]. On the other hand, Mendelian randomization (MR) studies reported that genetically elevated plasma HDL-C and LDL-C levels were positively associated with breast cancer risk [5,6,7], but some studies indicated that there was no association between genetically elevated plasma HDL-C and LDL-C levels and breast cancer risk [8]. Patients with dyslipidemia receive lipid-modifying agents such as 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins), but studies have not been able to exclude these effects. Furthermore, the distribution of risk factors for breast cancer and the incidence of breast cancer varies across countries [1, 9]; however, to the best of our knowledge, no large Japanese studies have comprehensively examined the association between cholesterol and breast cancer risk.
JMDC Inc. provides an epidemiological database that has accumulated claims and health checkup data from multiple health insurers in Japan, including a cumulative population of approximately 14 million people. In this study, we aimed to evaluate the association between cholesterol and breast cancer risk in Japanese women by using the database of health insurance claims and health checkup data.
Materials and methods
Data source and study participants
This study used the data of health insurance claims and health checkups collected by JMDC Inc., Tokyo, Japan. In Japan, all citizens are provided with a universal health insurance program, and each employer is required to provide its employees with insurance and regular health checkup opportunities. The JMDC Claims Database is an epidemiological claims database that has accumulated claims (inpatient, outpatient, and dispensing) and health checkup data from multiple health insurers since 2005. The cumulative population is approximately 14 million (as of February 2022), and the data enable the study of the prevalence and/or incidence rate of any disease in the general population, including healthy people, and can track hospital transfers or multiple facility visits. The JMDC Claims Database includes information on employee demographics, medical history, drug prescriptions, and hospital claims records based on International Classification of Diseases 10th Edition (ICD-10) coding.
We recruited 1,283,619 women who were insured between April 2008 and July 2019 and had at least one health checkup during that period. We excluded participants who had been insured for less than one year at the start of follow-up (n = 179,388) to ascertain cancer incidence and medication prescription status prior to the start of follow-up. We also excluded participants with no LDL-C, HDL-C, or triglyceride (TG) data (n = 29,499) and those with a breast cancer diagnosis prior to the start of follow-up (n = 4,523). To eliminate the influence of lipid-modifying agents, we excluded participants who had been prescribed lipid-modifying agents (WHO-ATC code: C10) at least once from 1 year before the start of follow-up to the end of follow-up (n = 114,050). The final analysis cohort consisted of 956,390 women.
Cholesterol levels and other covariates
Age, body mass index (BMI), blood pressure, and fasting laboratory values, including cholesterol and blood glucose levels, were collected at the time of health checkup by using a standardized protocol at each health care institution. Data on smoking status, alcohol consumption, and physical activity were collected at the time of the health checkup by using a self-administered questionnaire. Hormonal medication use was defined using the insurance claims of drug prescriptions.
On the basis of the cholesterol level at the time of the initial health checkup (the start of follow-up), the participants were classified into quintiles, and the group with the lowest cholesterol level was used as the reference. The following definitions were used for the other covariates [10]. Hypertension was defined as a systolic blood pressure of 140 mmHg or higher, diastolic blood pressure of 90 mmHg or higher, or use of antihypertensive drugs. Diabetes mellitus was defined as fasting blood glucose of 125 or higher or use of diabetes medication. Current smokers were participants who have had a total of 100 or more cigarettes or had smoked for 6 months or longer and also smoked in the last month. Physical activity was defined as ≥ 30 min of exercise for more than 2 times a week or more than 1 h of walking per day. Current hormone users were participants who were prescribed hormone drugs (WHO-ATC code: G03C estrogens, G03D progestogens, or G03F progestogens and estrogens in combination) at least once from one year before the start of follow-up to the start of follow-up. There were no data on hormonal contraceptives for systemic use (WHO-ATC code; G03A) in the JMDC Claims Database because they are not covered by insurance in Japan.
Case identification
Breast cancer was identified using an algorithm combining the diagnosis code for breast cancer (ICD-10 code: C500 to C506, C508, and C509), breast cancer–specific procedures, radiotherapy, and drugs. Details regarding breast cancer–specific procedures, radiotherapy, and drugs are reported elsewhere [11]. This algorithm has been shown to be a valid algorithm for identifying patients with newly diagnosed cancer from a Japanese claims database by comparison with the national cancer registry data [11]. In addition, other validation studies have been conducted in Japan to identify breast cancer by using a claims database, and the accuracy was reported to be high when the definition was combined disease codes and cancer treatment codes (surgery, chemotherapy, medication, and radiation procedure) [12, 13]. These studies also suggest that the Japanese claims database can accurately define the incidence of breast cancer.
The month of breast cancer incidence was defined as the month in which the ICD-10 codes for breast cancer, treatmen, and drugs were recorded in the claims in the same month during the insurance coverage period. The day of breast cancer incidence was defined as the 15th day of the month of breast cancer incidence.
Statistical analysis
The baseline characteristics of the participants are presented using means and standard deviations or medians and interquartile ranges for continuous variables and percentages for categorical variables.
The Cox proportional hazards regression model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) to describe the risk of breast cancer incidence associated with LDL-C, HDL-C, or TG. Person-years of follow-up for each participant were calculated from the date of the first health checkup and censored at the date of breast cancer incidence, withdrawal from insurance due to death or job change, or end of the study period (July 31, 2019) whichever occurred first. On the basis of the LDL-C, HDL-C, or TG level at the time of the initial health checkup (the start of follow-up), the participants were classified into quintiles, and the group with the lowest LDL, HDL, or TG level was used as the reference. P values for trends were calculated by assigning scores for each quintile category as an independent continuous variable in the model. Alternatively, they were divided by the clinical cutoff values for diagnosing hyperlipidemia (140 mg/ml for LDL-C, 40 mg/ml for HDL-C, and 150 mg/ml for TG), and the group with the lowest value was used as the reference. The analysis was adjusted for known breast cancer risk factors as confounders [14]. Model 1 was stratified by age group (< 50 and ≥ 50) and adjusted for age (continuous). Model 2 was stratified by age groups (< 50 and ≥ 50) and adjusted for age (continuous), BMI (< 18.5, 18.5–25, 25–30, or > 30 kg/m2), hypertension (yes or no), diabetes mellitus (yes or no), current smoker (yes, no, or missing), drinking status (daily, sometimes, rarely, or missing), physical inactivity (yes or no), and current hormone use (yes or no).
A stratified analysis by age group (< 50 and ≥ 50 years) was conducted using age 50 as a surrogate indicator of menopausal status to examine effect modification by menopausal status. P values for interaction were calculated by adding product terms for LDL-C, HDL-C, or TG and age groups (< 50 and ≥ 50) to the main models, with adjustments for the aforementioned confounders. We conducted a sensitivity analysis that excluded participants with a follow-up period of less than 5 years to prevent potential reverse causation and ensure a sufficient latent period.
All P values reported were two-sided, and the significance level was set at P < 0.05. Statistical analyses were performed using Stata (version 16.0; Stata Corporation, College Station, TX, USA).
Results
During the 2,832,277 person-years observation period (median: 2.4 years), 6284 participants were diagnosed with breast cancer. The crude incidence rate was 222 cases per 100,000 person-years. Table 1 shows the baseline characteristics of the study participants according to the quintile of LDL-C level at their first health checkup during the observation period. The highest LDL-C group had a higher average age and higher percentage of BMI (> 30), hypertension, diabetes, and physical activity than the lowest LDL-C group. However, the highest LDL-C group had a lower percentage of smokers, daily alcohol drinkers, and hormone users than the lowest LDL-C group. The observation period was slightly shorter for the participants with the highest LDL-C than those with the lowest LDL-C but did not differ substantially from the median (2.4 years) for all participants in either group.
Table 2 shows the HRs of incidence for breast cancer according to the quintile of LDL-C, HDL-C, and TG. Model 2 showed an increased risk of breast cancer in the highest LDL-C group compared with the lowest LDL-C group [HRQ1 vs. Q5 = 1.08 (95% CI 0.99–1.18), P-trend = 0.273] with marginal statistical significance. In an analysis at the clinical cutoff values for diagnosing hyperlipidemia (140 mg/ml), the risk of breast cancer was also increased [HR<140 mg/ml vs. ≥140 mg/ml = 1.09 (95% CI 1.02–1.16)]. There was no association between HDL-C or TG and breast cancer in either quintile or the clinical cutoff values for diagnosing hyperlipidemia [HRQ1 vs. Q5 = 0.98 (95% CI 0.90–1.06), P-trend = 0.307; HR<40 mg/dl vs. ≥40 mg/dl = 1.22 (95% CI 0.91–1.65) for HDL-C; HRQ1 vs. Q5 = 1.02 (95% CI 0.94–1.11), P-trend = 0.745; HR<150 mg/dl vs. ≥150 mg/dl = 0.99 (95% CI 0.89–1.10) for TG].
Lipid abnormalities may be present even before cancer is detected because they are the most prominent metabolic abnormalities in cancer [15]. In addition, breast cancer requires several years to develop [16]. To prevent potential reverse causation and ensure a sufficient latent period, we performed sensitivity analysis only on participants with an observation period of at least five years (Table S1). The results showed a positive association between LDL-C and breast cancer risk, similar to that in the main analysis. The HR estimate was slightly larger than in the main analysis and was statistically significant [HRQ1 vs. Q5 = 1.32 (95% CI 1.03–1.69), P-trend = 0.185; HR<140 mg/ml vs. ≥140 mg/ml = 1.24 (95% CI 1.02–1.50)]. There were no differences in HDL-C and TG levels, similar to that in the main analysis.
Breast cancer has distinct causes and prognoses in patients of premenopausal and postmenopausal age [1]. To examine the differences in the effects of LDL-C, HDL-C, and TG on the risk of breast cancer between premenopausal and postmenopausal women, we performed a stratified analysis. Given that there were no data on menopausal status in the JMDC Claims Database, we referred to the average age of menopause in Japan as 50 years [1, 17] and treated participants under and over 50 years old as premenopausal and postmenopausal, respectively. The crude incidence rate was lower in women under 50 years of age (197 cases per 100,000 person-years) than in women over 50 years of age (297 cases per 100,000 person-years). In women under 50 years old, the highest LDL-C group had increased breast cancer risk [HR Q1 vs. Q5 = 1.13 (95% CI 1.02–1.25), P-trend = 0.715] compared with the lowest LDL-C group, whereas there was no association in women over 50 years old [HR Q1 vs. Q5 = 0.94 (95% CI 0.79–1.12), P-trend = 0.469] (Table 3). Furthermore, the risk of breast cancer was significantly increased in an analysis using the clinical cutoff values for diagnosing hyperlipidemia [HR <140 mg/ml vs. ≥140 mg/ml = 1.15 (95% CI 1.06–1.26)] in women under 50 years old, but there was no difference in women over 50 years old [HR <140 mg/ml vs. ≥140 mg/ml = 1.04 (95% CI 0.94–1.14)] (Table 3). The interaction of age group (< 50 and ≥ 50 years) was statistically significant in both analyses (P-interaction = 0.017 and 0.008). In a sensitivity analysis with a five-year latent period (Table S2), there was an increased risk of breast cancer incidence in women under 50 years old [HR Q1 vs. Q5 = 1.39 (95% CI 1.06–1.82), P-trend = 0.077; HR <140 mg/ml vs. ≥140 mg/ml = 1.31 (95% CI 1.05–1.64)]. No difference was found in women over 50 years of age (Table S2).
In the stratified analysis of age groups (< 50 and ≥ 50) (Table 3), there was a significant inverse association between HDL-C and breast cancer in women over 50 years old [HR Q1 vs. Q5 = 0.84 (95% CI 0.73–0.97), P-trend = 0.025]; however, there was no association in women under 50 years old [HR Q1 vs. Q5 = 0.96 (95% CI 0.87–1.06), P-trend = 0.268], with a marginally statistically significant interaction of age groups (< 50 and ≥ 50). In a sensitivity analysis with a five-year latent period (Table S2), the reduction in the risk of breast cancer incidence in women over 50 years old was attenuated in terms of statistical significance [HR Q1 vs. Q5 = 0.76 (95% CI 0.45–1.30), P-trend = 0.833], but the effect estimates remained similar compared with the main analysis. The analysis at the clinical cutoff values for diagnosing hyperlipidemia showed no difference in either group, and there were wide CIs for the estimates because of the very small number of breast cancer cases among participants with HDL-C < 40 mg/ml (Table 3).
In the stratified analysis of age groups (< 50 and ≥ 50) (Table 3), there was some evidence of a positive association between TG and breast cancer in women over 50 years [HR Q1 vs. Q5 = 1.15 (95% CI 0.98–1.35), P-trend = 0.033, P-interaction = 0.292]. However, there was no statistically significant difference when the lowest TG group was compared with the highest TG group, and there was no statistically significant interaction by age group. There was no association between TG and breast cancer in women under 50 years [HR = 0.98 (95% CI 0.88–1.09), P-trend = 0.639]. Sensitivity analysis with a five-year latent period (Table S2) showed an increase in the point estimate of the HR, but there was no statistically significant difference in women over 50 years [HR Q1 vs. Q5 = 1.63 (95% CI 0.75–3.56), P-trend = 0.598]. The analysis of the clinical cutoff values for diagnosing hyperlipidemia showed no difference in either group (Table 3).
Discussion
This cohort study analyzed the health insurance claims and health checkup data of more than 950,000 women in Japan and found that women with LDL-C ≥ 140 mg/ml had a modest but significantly increased breast cancer risk compared with women with LDL-C < 140 mg/ml.
This study showed a modestly positive association between LDL-C and breast cancer risk [HR Q1 vs. Q5 = 1.08 (95% CI 0.99–1.18), P-trend = 0.273; HR <140 mg/ml vs. ≥140 mg/ml = 1.09 (95% CI 1.02–1.16)]. Previous meta-analyses of observational studies have found no association between LDL-C and breast cancer risk [3, 4]. They were conducted mainly in Europe and America, with few Japanese data. The limitation of these studies is that the effects of lipid-modifying agents, including statins, which significantly alter serum LDL-C levels, have not been ruled out. A recent MR study reported that genetically elevated LDL-C increases the risk of breast cancer [5,6,7]. Proliferating cancer cells have an increased requirement for cholesterol, thus increasing the expression of the LDL-C receptor and uptake of LDL-C into breast cancer tissues [18]. The metabolites of lipid peroxide cause conformational changes in deoxyribonucleic acid (DNA) and reduce DNA repair capacity. Delimaris et al. [19] reported that breast cancer patients have elevated serum levels of oxidized LDL-C and that serum levels of oxidized LDL-C are associated with increased breast cancer risk. Given these findings, the results obtained in the present study indicate that LDL-C increases breast cancer risk.
Statins reduce serum LDL-C. A meta-analysis examining the association between statins and breast cancer risk did not find an association between statins and breast cancer risk, although there was significant heterogeneity between the estimates [20]. However, stratified analyses showed that statins have some breast cancer preventive effect in Asians compared with Americans and in long-term statin users compared with short-term statin users. Breast cancer rates vary widely by country [1, 9]; therefore, studies of the association between LDL-C, statins, and breast cancer may need to be restricted to Asians, including the Japanese.
In this study, no association was found between serum HDL-C levels and the risk of breast cancer. However, among women over 50 years of age, those with higher HDL-C levels may have a lower risk of breast cancer. Previous meta-analyses have reported that higher HDL-C levels decreases the risk of postmenopausal breast cancer [3, 4], and this finding is similar to that of the present study. HDL-C has antioxidant and anti-inflammatory effects, inhibits the LDL-C oxidation cascade. Furthermore, apolipoprotein A-I, which is the main component of HDL-C in serum, inhibits cell proliferation [18]. These mechanisms suggest that HDL suppresses breast cancer development. The differential effect of HDL-C on breast cancer risk depending on menopausal status is difficult to interpret. Ni et al. [3] suggested that low HDL-C indicates relative androgen deficiency and that breast cancer risk in postmenopausal women may be explained by the association between androgens and breast cancer risk. By contrast, a large population-based cohort study in Japan showed that subjects with HDL-C ≥ 40 mg had an increased risk of breast cancer compared with subjects with HDL-C ≤ 40 mg [21]. In the present study, participants with HDL-C < 40 mg/ml accounted for 0.87% (n = 8341) of the total participants (n = 956,390). The small number of breast cancer cases (n = 44) limited the statistical power of the analysis at the clinical cutoff values for diagnosing hyperlipidemia.
In this study, no association was found between TG and risk of breast cancer, although there was some evidence of a positive association between TG and breast cancer in women over 50 years [HR Q1 vs. Q5 = 1.15 (95% CI 0.98–1.35), P-trend = 0.033, P-interaction = 0.292]. Previous reports showed that there was no association between TG and breast cancer [5], but others showed an inverse association between TG and breast cancer [3, 8]. Studies on the mechanisms linking TG and breast cancer are scarce. Therefore, the association between TG levels and breast cancer needs to be further investigated in detail.
This study has several strengths. First, the analysis was based on a large individual-traceable database. Second, the outcome (breast cancer incidence) was identified by a highly validated definition from health insurance claims data. Third, the analysis excluded individuals taking lipid-modifying agents, such as statins, thus allowing for the estimation of effects that exclude the influence of these drugs. In other words, the analysis was conducted in a population whose exposure (cholesterol or triglyceride levels) was relatively stable during the observation period. This study had several limitations. First, important risk factors for breast cancer (e.g., age at menarche, genetic factors, family history of breast cancer, fat intake, parturition, lactation status, and contraception) [14] may have confounded the effect of cholesterol. These factors were not available in the JMDC Claims Database and could not be adjusted in the multivariable analysis. Second, breast cancer cannot be divided into subtypes according to receptor expression status. Given that nonclinical studies have shown that the effect of LDL-C and HDL-C on breast cancer development is dependent on receptor expression status [18], the effect of cholesterol on breast cancer may vary by subtype even in a clinical setting. Third, the observation period for this study was relatively short (median: 2.4 years), considering that breast cancer has a long-term induction and latency period. However, a sensitivity analysis restricted to participants with an observation period > 5 years yielded results with a similar trend.
In conclusion, this study showed the modest association of LDL-C at the clinical cutoff values for diagnosing hyperlipidemia (140 mg/mL) and indicated that HDL-C and TG had no associations with breast cancer risk in Japanese women.
Data availability
The database used in this study is available for purchase from JMDC Inc., Tokyo, Japan (https://www.jmdc.co.jp/en/).
References
Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM (2020) Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health 8(8):e1027–e1037
Kuzu OF, Noory MA, Robertson GP (2016) The role of cholesterol in cancer. Cancer Res 76(8):2063–2070
Ni H, Liu H, Gao R (2015) Serum lipids and breast cancer risk: a meta-analysis of prospective cohort studies. PLoS ONE 10(11):e0142669
Touvier M, Fassier P, His M, Norat T, Chan DS, Blacher J et al (2015) Cholesterol and breast cancer risk: a systematic review and meta-analysis of prospective studies. Br J Nutr 114(3):347–357
Nowak C, Ärnlöv J (2018) A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun 9(1):3957
Qi G, Chatterjee N (2019) Mendelian randomization analysis using mixture models for robust and efficient estimation of causal effects. Nat Commun 10(1):1941
Johnson KE, Siewert KM, Klarin D, Damrauer SM, Program VAMV, Chang KM et al (2020) The relationship between circulating lipids and breast cancer risk: a Mendelian randomization study. PLoS Med 17(9):e1003302
Orho-Melander M, Hindy G, Borgquist S, Schulz CA, Manjer J, Melander O et al (2018) Blood lipid genetic scores, the HMGCR gene and cancer risk: a Mendelian randomization study. Int J Epidemiol 47(2):495–505
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Itoh H, Kaneko H, Okada A, Yano Y, Morita K, Seki H et al (2021) Fasting plasma glucose and incident colorectal cancer: analysis of a Nationwide Epidemiological Database. J Clin Endocrinol Metab 106(11):e4448–e4458
Ogawa T, Takahashi H, Saito H, Sagawa M, Aoki D, Matsuda K et al (2023) Novel algorithm for the estimation of cancer incidence using claims data in Japan: a feasibility study. JCO Glob Oncol 9:e2200222
de Luise C, Sugiyama N, Morishima T, Higuchi T, Katayama K, Nakamura S et al (2021) Validity of claims-based algorithms for selected cancers in Japan: results from the VALIDATE-J study. Pharmacoepidemiol Drug Saf 30(9):1153–1161
Sato I, Yagata H, Ohashi Y (2015) The accuracy of Japanese claims data in identifying breast cancer cases. Biol Pharm Bull 38(1):53–57
Momenimovahed Z, Salehiniya H (2019) Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press) 11:151–164
Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z (2021) Lipid metabolism and cancer. J Exp Med. https://doi.org/10.1084/jem.20201606
Umar A, Dunn BK, Greenwald P (2012) Future directions in cancer prevention. Nat Rev Cancer 12(12):835–848
Kono S, Sunagawa Y, Higa H, Sunagawa H (1990) Age of menopause in Japanese women: trends and recent changes. Maturitas 12(1):43–49
Cedo L, Reddy ST, Mato E, Blanco-Vaca F, Escola-Gil JC (2019) HDL and LDL: potential new players in breast cancer development. J Clin Med 8(6):853
Delimaris I, Faviou E, Antonakos G, Stathopoulou E, Zachari A, Dionyssiou-Asteriou A (2007) Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin Biochem 40(15):1129–1134
Islam MM, Yang HC, Nguyen PA, Poly TN, Huang CW, Kekade S et al (2017) Exploring association between statin use and breast cancer risk: an updated meta-analysis. Arch Gynecol Obstet 296(6):1043–1053
Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, Iso H et al (2009) Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev 18(3):240–247
Acknowledgements
The authors would like to express their deep gratitude to JMDC Inc., which licensed the use of the JMDC Claims Database for this research.
Funding
Open access funding provided by Osaka University. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
NN and TS designed the work that led to the submission. NN and LZ performed statistical analyses. All authors participated in the interpretation of the data. NN drafted the manuscript. All authors critically revised the manuscript, approved the final manuscript as submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethical Review Board of Osaka University, Osaka, Japan [20490].
Consent to participate and publish
The need for informed consent and consent to publish was waived owing to the retrospective nature of the study and the anonymity of the patient database.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Narii, N., Zha, L., Komatsu, M. et al. Cholesterol and breast cancer risk: a cohort study using health insurance claims and health checkup databases. Breast Cancer Res Treat 199, 315–322 (2023). https://doi.org/10.1007/s10549-023-06917-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06917-z