Abstract
Purpose
Neoadjuvant endocrine therapy (NET) is a treatment option for estrogen receptor-positive (ER+) postmenopausal early breast cancer (EBC). This phase III trial evaluated the prognosis of EBC patients treated with/without chemotherapy (CT) following NET.
Methods
ER+/HER2−, T1c-2, and clinically node-negative EBC patients were enrolled in 2008–2013 and treated with endocrine therapy (ET) in weeks 24–28. All patients, excluding those with progressive disease (PD) during NET or ≥ 4 positive lymph nodes after surgery, were randomized to ET for 4.5–5 years with/without CT. The primary endpoint was disease-free survival (DFS). Secondary endpoints included distant DFS (DDFS), overall survival (OS), and DFS/DDFS/OS according to clinical response to NET.
Results
Of 904 patients, 669 were randomized to CT+ET (n = 333) or ET alone (n = 336). The median follow-up was 7.8 years. DFS (CT+ET, 47 events; ET alone, 70 events) and DDFS did not reach the planned numbers of events. Eight-year DFS/DDFS rates were 86%/93% and 83%/92%, respectively. DFS was significantly better in CT+ET than ET alone in subgroups aged < 60 years (P = 0.016), T2 (P = 0.013), or Ki67 > 20% (P = 0.026). Progesterone receptor and histological grade were predictive markers for clinical responses to NET.
Conclusion
NET may be used as standard treatment for patients with ER+EBC. Although it is difficult to decide whether to administer adjuvant CT based solely on the effect of NET, the response to NET may help to inform this decision.
Trial registration
This study was registered at the UMIN Clinical Trials Registry under UMIN000001090 (registered 20 March 2008).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most commonly diagnosed cancers worldwide [1]. Hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative disease accounts for more than 70% of incident breast cancer patients [2]. In the past three decades, survival outcomes of patients with early breast cancer (EBC) have notably improved, mainly due to early detection of the disease and advances in adjuvant treatments, such as endocrine therapy (ET), chemotherapy (CT), and anti-HER2 therapy. ET is the standard of care after surgery in patients with estrogen receptor-positive (ER+) breast cancer (luminal breast cancer) and current clinical data recommend longer treatment with ET in these patients [3, 4].
The St. Gallen conference in 2007 recommended ET alone and CT followed by ET for patients with highly or incompletely endocrine-responsive and HER2−, intermediate-risk breast cancer [5]. Many investigators have discussed the need for adjuvant CT in patients with ER+/HER2−, node-negative early-stage breast cancer, according to the multiple prognostic gene signature and other methods [6].
Recurrence score (RS), determined by a 21-gene signature, shows promise for identifying high-risk patients among patients with luminal breast cancer. The TAILORx study showed that adjuvant ET and chemoendocrine therapy had similar efficacies in postmenopausal women with ER+, HER2−, axillary node-negative breast cancer who had a mid-range RS, excluding patients aged ≤ 50 years [7]. The same study also reported a good prognosis in patients with a RS < 11, even without CT [8]. However, the 21-gene signature is costly to determine and is not covered by health insurance in many countries, and novel strategies are therefore required to determine the need for postoperative CT in patients with luminal node-negative breast cancer.
We therefore conducted a randomized phase III study (NEOS) to assess the long-term prognosis of patients with ER+EBC treated with neoadjuvant ET (NET) with/without adjuvant CT [6]. We previously reported the impact of NET on the health-related quality of life during NET and confirmed the feasibility of NET in patients with EBC [9]. In addition, we demonstrated that the 21-gene RS in core needle samples could be a predictive responsive marker of NET in the TransNEOS study [10]. Here we present the primary results of the NEOS trial with a longer median follow-up duration of 7.8 years.
Methods
Detailed methods can be found in the study protocol (Supplementary file 1).
Study design
This was an open-label, randomized, parallel-group controlled study involving patients responding to NET. Between 16 May 2008 and 7 June 2013, 904 patients from 100 institutions in Japan were enrolled and treated with letrozole (LET). The study consisted of preoperative and postoperative treatment periods [6].
Patients
Preoperative enrollment
Postmenopausal women aged < 76 years with histologically diagnosed primary invasive ER+/HER2−, T1c-T2, N0 and M0 breast cancer were enrolled. The definition of ER+was ≥ 10% of cells stained by immunohistochemical assay at each local site in a pretreatment needle biopsy specimen. Patients with proven metastasis to a sentinel lymph node, synchronous or asynchronous bilateral breast cancer, multiple tumors located in multiple breast segments, double primary invasive cancer untreated or diagnosed within 5 years after completing the treatment for the previous cancer, a history of breast cancer, or ongoing treatment with any continuous systemic corticosteroid, any estrogen-containing agent, or any selective ER modulator were ineligible.
Postoperative enrollment
Patients with a clinical response to LET including complete response (CR), partial response (PR), or stable disease (SD), and who completed breast cancer surgical treatment, with lymph node-negative or -positive disease (1–3 nodes) were enrolled. If the primary tumor did not show progressive disease (PD), patients with 1–3 lymph node metastases were included in the randomized cohort without judging them as PD, assuming that they had metastasis from the beginning. Patients with HER2+ in a surgical specimen were excluded.
Randomization and masking
Enrolled patients were randomized to receive either CT followed by LET or LET alone at an approximate ratio of 1:1 by dynamic allocation with stratification by the following factors: response to neoadjuvant LET therapy (CR or PR vs. SD), progesterone receptor (PgR) status at primary enrollment (positive vs. negative), pathological node status (positive vs. negative), age at primary enrollment (< 60 vs. ≥ 60 years), and study center.
Procedures
During the preoperative period, eligible patients started a 24–28-week course of NET comprising once daily oral intake of 2.5 mg/day LET, within 4 weeks after enrollment. The clinical response to LET was evaluated at 1, 2, and 4 months after the start of the treatment by clinical examination and ultrasonography (US), and at the conclusion of treatment at 6 months by clinical examination, US, and either computed tomography or magnetic resonance imaging (MRI). The clinical response evaluation was only done for the primary tumor, and not for the lymph node area. If PD was identified, LET was discontinued and patients were treated and followed up in accordance with the pre-determined procedures.
Surgery for breast cancer was performed 1–4 weeks after the completion of NET. Patients who were eligible in the postoperative period were randomized to receive either CT followed by LET (CT+ET group) or LET alone (ET group) at a 1:1 ratio within 4 weeks after postoperative enrollment for 4.5–5 years. The treatment regimen for LET in both groups was the same as the regimen during the preoperative period.
Outcomes
The primary endpoint was disease-free survival (DFS), defined as the time from the date of first registration until the date of the first event (recurrence in the ipsilateral preserved breast, ipsilateral chest wall or regional lymph node, or distant organ metastasis; secondary cancer without cutaneous basal cell carcinoma/spindle cell carcinoma and uterine carcinoma in situ; or all-cause death) in each group (CT+ET and ET). Secondary endpoints were the percentage of patients clinically responding to NET (CR or PR) and histological tumor response to NET, and DFS, distant DFS (DDFS), and overall survival (OS) in each group. Other secondary endpoints were the percentages of patients undergoing breast-conserving surgery, safety, health-related quality of life, and cost-effectiveness. DDFS was defined as the time to recurrence in distant organs such as the bone, liver, lung, brain excluding soft tissue or locoregional region, and breast cancer-unrelated death since randomization. OS was defined as the time from the date of primary enrollment until the date of death from any cause. Clinical staging, histological classification, and clinical response to NET were assessed in accordance with the General Rules for Clinical and Pathological Recording of Breast Cancer 15th Edition [11]. The Japanese version of the Eastern Cooperative Oncology Group scale was also used to grade performance status [12]. Clinical response was evaluated by inspection/palpation and US at each specified time point, and computed tomography or MRI was performed at the completion of NET. ER, PgR, and HER2 statuses and nuclear grade as eligibility criteria were assessed by local pathologists.
Statistical analysis
The aim of this study was to determine if patients who responded to NET should choose ET alone or CT+ET as adjuvant therapy, in terms of DFS. To prepare for this analysis, all centers scheduled to participate in this study (n = 100) were sent a questionnaire and 78 of them responded. The results of the questionnaire survey were as follows: the mean predicted 5-year DFS with ET alone was 85% and the mean highest 5-year DFS with CT+ET that would strongly discourage oncologists to add adjuvant CT was 87% (condition A), whereas the mean lowest 5-year DFS with CT+ET that would strongly encourage oncologists to add adjuvant CT was 92% (condition B). Assuming an exponential distribution of DFS, the expected hazard ratios (HRs) for CT+ET relative to ET alone under conditions A and B were calculated to be 0.90 and 0.52, respectively.
Based on these survey results, the HR thresholds for choosing between the two treatments under conditions A and B were set at 0.9 and 0.6, respectively. A total of about 200 events were needed to provide a statistical power of 90% with these thresholds. Assuming that the 5-year DFS in the entire population was 88% and that 90% of the subjects would show CR, PR, or SD to NET, about 1,460 patients were needed to observe an occurrence of about 200 events during the planned follow-up of up to 8 years (a 3-year enrollment period plus a 5-year follow-up period). Accounting for an expected withdrawal of about 10% of the subjects, 1,700 patients were planned to be enrolled.
Patient enrollment did not proceed on schedule and the Study Steering Committee proposed postponing the enrollment period from 3 to 5 years and the total study period from 8 to 10 years in March 2011, which was approved by the Independent Data Monitoring Committee. We set the selection probability as 90%, but it was judged that it was possible to almost achieve the purpose of this study at about 80%–85%, in which case, about 170 events were required for both groups. In addition, assuming that the 5-year DFS for the overall population was 88%, the durations of the accrual and follow-up periods were 5 and 10 years (15 years for the longest follow-up period), respectively. A total sample size of 630 patients was required. Assuming that about a quarter of the preoperative patients were not registered in the second (postoperative) stage, the total sample size required was about 850 patients. Kaplan–Meier curves for DFS, DDFS, and OS were estimated for each response group. Median 8-year DFS/OS/DDFS with confidence intervals (CIs) were calculated based on the Greenwood’s formulae. A Cox’s proportional hazard model was used to investigate the relationships between clinical response to ET (CR, PR, or SD versus PD), DFS, DDFS, and OS. HRs with 95% CIs were obtained for DFS, DDFS, and OS. Logistic regression was used to investigate the relationships between predictive variables (age, body mass index, histological grade, Ki67, PgR, T, and HER2) and clinical responses. Given that this analysis was not performed at the pre-planned time and because further extension of the observation period would not achieve the planned event, we consulted with a biostatistician and decided to perform the analysis, even though the primary endpoint could not be proven with statistical certainty.
Results
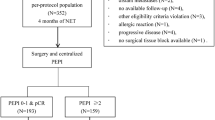
A total of 904 patients from 100 institutions in Japan were enrolled between 16 May 2008 and 7 June 2013, and treated with LET (Fig. 1). This study was terminated early because the number of events was unlikely to reach the planned number even after further observation. Among the 904 patients, 22 patients were withdrawn during NET because of patient request (15 patients), ineligibility (2 patients), transfer to another hospital (2 patients), and unknown reasons (3 patients). Among the remaining 882 patients who received NET, 15 patients (2%) showed CR, 422 (48%) showed PR, 403 (46%) showed SD, and 42 (5%) showed PD. The percentage of patients with a clinical response to NET (CR or PR) was 50% (437/882 patients). All PD patients had undergone complete resection of all residual disease following NET. Prior to randomization, 171 patients were excluded because they did not meet the postoperative enrollment criteria (65 patients: HER2+ in 8 patients; ≥ 4 positive lymph nodes in 8 CR or PR patients, 34 SD patients, and 8 SD patients with nuclear grade 3/vascular invasion, and other reasons in 7 patients), completion of the preoperative treatment in < 24 weeks for reasons other than PD (11 patients), refusal of surgery or postoperative protocol treatment (54 patients), patient’s preference (12 patients), other reasons (27 patients), and unknown reasons (2 patients). The 669 patients with a clinical response to NET of CR, PR, or SD and who completed breast cancer surgical treatment were assigned 1:1 to CT+ET or ET alone. The patients’ characteristics (age, tumor size, PgR status, nuclear grade, clinical and pathological responses to NET, and number of axillary lymph node metastases) were well balanced between the two groups (Table 1). The PgR+ and Ki67 < 20% rates were 81% and 60% in both groups, respectively. The CT regimens received by patients in the CT+ET group are summarized in Supplementary Table S1.
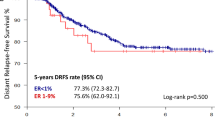
The median duration of follow-up was 7.8 years (range 0.1–19.6 years). Formal analysis of the primary end point was not possible because there were fewer DFS events (CT+ET 47, ET alone 70) than planned (HR 0.74 [95% CI 0.51, 1.09]) (Table 2, Fig. 2A). Similarly, there was no formal analysis of DDFS for the same reason (HR 0.79 [95% CI 0.46, 1.35]) (Fig. 2B). The DFS/DDFS rates at 8 years were 86%/93% and 83%/92% in the CT+ET and ET alone groups, respectively (Fig. 2A, B). There was no significant difference in OS between the two groups (HR 0.46 [95% CI 0.20, 1.07]) (Fig. 2C). DFS was significantly better in the CT+ET than in the ET alone group for some subgroups (Fig. 3): age < 60 years (HR 0.37, P = 0.016), clinical T2 (HR 0.56, P = 0.013), and Ki67 ≥ 20% patients (HR 0.49, P = 0.026) at baseline (Supplementary Fig. S1). However, there was no difference in DFS between CT+ET and ET alone in patients aged ≥ 60 years (HR 0.94, P = 0.78), T1c (HR 1.58, P = 0.23), and Ki67 < 20% (HR 1.06, P = 0.83) (Supplementary Fig. S2). There was no significant difference in DFS between the two groups according to response to NET (HR 0.75, P = 0.28 in CR and PR groups; HR 0.74, P = 0.29 in SD group) (Supplementary Fig. S3). Predictive markers of a clinical response to LET were determined by comparing clinical and pathological factors among patients with CR, PR, SD, and PD. Multivariate analyses showed that PgR (+ vs. −) and histological grade (3 vs. 1), but no other factors (age, body mass index, T, Ki67, or HER2), were markers of a clinical response to LET (Supplementary Table S2). No new adverse events were observed during NET and adjuvant therapy.
Discussion
To the best of our knowledge, this was the first study to assess the value of response-guided therapy using NET. We found no difference in survival outcomes of patients with ER+EBC treated with NET, excluding patients with PD, compared with previous data for the same population [13]. Capecitabine and trastuzumab emtansine are standard treatments for patients with residual triple-negative and HER2+ breast cancer after neoadjuvant standard treatment, respectively, based on escalation studies in patients with non-pathological clinical response after neoadjuvant CT as the standard regimen [14, 15]. Response-guided therapy using neoadjuvant CT is currently a standard of care for patients with residual cancer with TN and HER2+. However, no de-escalation studies have excluded CT after neoadjuvant treatment for EBC, regardless of subtype. Furthermore, there are no confirmed data on NET-response-guided therapy for luminal-type breast cancer.
We considered that patients with luminal-type EBC who had PD following NET might have worse outcomes than those with CR, PR, and SD, based on the poorer prognosis of patients with endocrine primary resistance compared with those with a good response in patients with ER+metastatic breast cancer, and we therefore planned a randomized controlled trial to compare CT+ET and ET alone in patients with CR, PR, and SD, excluding PD, after NET. Previous small studies found different response outcomes to ET [16, 17].
The prognostic value of the clinical response to NET has previously been examined in small-scale studies [16, 17]. The correlation between tumor shrinkage by NET and survival has been reported in several studies [16, 17]. CR rates are generally lower in patients treated with NET than in those with neoadjuvant CT [18, 19]. Indeed, the CR rate was only 2%.
The results of this study show that it is difficult to decide whether to administer adjuvant CT based solely on the effect of NET. Among patients with CR, PR, or SD (excluding PD) following NET, the CT+ET group had better outcomes compared with the ET alone group, as demonstrated by the 8-year DFS/DDFS rates, especially among patients aged < 60 years or with clinical T2 or high proliferation. In a previous report, tumor size and high proliferation factors were shown to be predictive markers for benefit from CT [5, 20]. In contrast, however, the current results suggested that adjuvant CT may not be needed in patients with CR, PR, and SD by NET with clinical T1c or low proliferation at baseline. These results warrant further studies to determine the benefits of CT in patients who respond to NET.
The definitions of PR and SD used in this study were equivalent to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, and a reduced tumor size ≤ 30% and an increased tumor size ≤ 20% were both classified as SD. However, the possible differences in DFS and DDFS rates between these two groups in patients with SD should be investigated in future studies.
In this study DFS/DDFS were significantly better in the CT+ET compared with the ET alone group in patients aged < 60 years. The benefit of additional CT in patients aged < 60 years might be a result of the CT regimen, with more patients aged > 60 years receiving the cyclophosphamide, methotrexate, and fluorouracil regimen compared with the docetaxel and cyclophosphamide regimen (data not shown).
A previous study showed that Ki67 down-regulation at an early phase during NET and a preoperative endocrine prognostic index score were predictive markers for survival benefit in patients with ER+EBC [21,22,23,24]. However, it is currently unclear if these factors can determine the need for adjuvant CT.
Based on the results of a large-scale trial [7], multigene assays have been used to decide on the need for adjuvant CT. Adjuvant CT is not recommended in patients with postmenopausal EBC with pathological negative to three positive nodes with estimated low or intermediate RS [7, 25]. However, a multigene assay may not be a convenient tool because of its high cost. In contrast, NET is a convenient strategy worldwide because of its lower cost. In the TransNEOS study, using the same population as the current study, we validated the use of the RS to predict clinical response to NET [10]. In patients with T2 tumors and high proliferation at baseline, we recommend using the multigene assay before NET using a core needle sample. The response to NET may help to inform the decision of whether to use adjuvant CT.
No patients in this study experienced any serious adverse events or discontinued the study due to an adverse event during NET (data not shown), indicating that NET was safe and well-tolerated in these patients. The results of our previous study demonstrating that NET had no impact on health-related quality of life in EBC patients [9] further support our present findings on the safety and tolerability of NET using LET.
This study had some limitations, including low power due to the low incidence of DFS events, with 8-year DFS and OS rates of > 90% and > 95%, respectively, in the overall population, regardless of the relatively long follow-up period (7.8 years). There were many patients in this study who did not receive the prescribed treatment after randomization. In particular, 67 of the patients assigned to CT+ET did not receive chemotherapy (Supplementary Table S1). Of those who did receive chemotherapy, 63 received cyclophosphamide, methotrexate, and 5-fluorouracil (Supplementary Table S1). This may be one of the reasons why we could not show the benefit of receiving chemotherapy.
Conclusion
NET may be used as standard treatment for patients with ER+EBC. Although it is difficult to decide whether to administer adjuvant CT based solely on the effect of NET, the response to NET may help to inform this decision.
Data availability
The data underlying the results presented in the study are available from CSPOR data center after publication (no end date). Data include individual de-identified participant data, a data dictionary as well as clinical trial protocols. Some restrictions apply due to confidentiality of patient data. Because these data are derived from a prospective clinical trial with ongoing follow-up collection there are legal and ethical restrictions to sharing sensitive patient-related data publicly. Data will be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Data can be requested in context of a research project sent to the corresponding author. Research proposals are approved by the NEOS steering committee.
Abbreviations
- CI:
-
Confidence interval
- CR:
-
Complete response
- CT:
-
Chemotherapy
- DDFS:
-
Distant disease-free survival
- DFS:
-
Disease-free survival
- EBC:
-
Early breast cancer
- ET:
-
Endocrine therapy
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- LET:
-
Letrozole
- MRI:
-
Magnetic resonance imaging
- NET:
-
Neoadjuvant endocrine therapy
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PgR:
-
Progesterone receptor
- PR:
-
Partial response
- RS:
-
Recurrence score
- SD:
-
Stable disease
- US:
-
Ultrasonography
References
International Agency for Research on Cancer. World Health Organization (2020) Globocan 2020. All cancers excluding non-melanoma skin cancers fact sheet. https://gco.iarc.fr/today/data/factsheets/cancers/40-All-cancers-excluding-non-melanoma-skin-cancer-fact-sheet.pdf. Accessed 13 June 2020
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LAG, Cronin KA (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2. J Natl Cancer Inst 106:dju055. https://doi.org/10.1093/jnci/dju055
Strasser-Weippl K, Badovinac-Crnjevic T, Fan L, Goss PE (2013) Extended adjuvant endocrine therapy in hormone-receptor positive breast cancer. Breast 22 Suppl 2:S171–S175. https://doi.org/10.1016/j.breast.2013.07.033
Davies C, Pan H, Godwin J et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–816. https://doi.org/10.1016/S0140-6736(12)61963-1
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn H-J (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18:1133–1144. https://doi.org/10.1093/annonc/mdm271
Iwata H (2011) Neoadjuvant endocrine therapy for postmenopausal patients with hormone receptor-positive early breast cancer: a new concept. Breast Cancer 18:92–97. https://doi.org/10.1007/s12282-010-0233-6
Sparano JA, Gray RJ, Makower DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379:111–121. https://doi.org/10.1056/NEJMoa1804710
Sparano JA, Gray RJ, Makower DF et al (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005–2014. https://doi.org/10.1056/NEJMoa1510764
Taira N, Iwata H, Hasegawa Y, Sakai T, Higaki K, Kihara K, Yamaguchi T, Ohsumi S, Shimozuma K, Ohashi Y (2014) Health-related quality of life and psychological distress during neoadjuvant endocrine therapy with letrozole to determine endocrine responsiveness in postmenopausal breast cancer. Breast Cancer Res Treat 145:155–164. https://doi.org/10.1007/s10549-014-2935-5
Iwata H, Masuda N, Yamamoto Y et al (2019) Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat 173:123–133. https://doi.org/10.1007/s10549-018-4964-y
Sakamoto G, Inaji H, Akiyama F, Haga S, Hiraoka M, Inai K, Iwase T, Kobayashi S, Sakamoto G, Sano M, Sato T, Sonoo H, Tsuchiya S, Watanabe T, Japanese Breast Cancer Society (2005) General rules for clinical and pathological recording of breast cancer 2005. Breast Cancer 12(Suppl):S1-27
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF, ATAC/LATTE investigators (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11:1135–1141. https://doi.org/10.1016/S1470-2045(10)70257-6
Masuda N, Lee SJ, Ohtani S et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376:2147–2159. https://doi.org/10.1056/NEJMoa1612645
von Minckwitz G, Huang CS, Mano MS et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617–628. https://doi.org/10.1056/NEJMoa1814017
Akashi-Tanaka S, Omatsu M, Shimizu C, Ando M, Terada K, Shien T, Kinoshita T, Fujiwara Y, Seki K, Hasegawa T, Fukutomi Y (2007) Favorable outcome in patients with breast cancer in the presence of pathological response after neoadjuvant endocrine therapy. Breast 16:482–488. https://doi.org/10.1016/j.breast.2007.02.003
Ueno T, Saji S, Masuda N, Kuroi K, Sato N, Takei H, Yamamoto Y, Ohno S, Yamashita H, Hisamatsu K, Aogi K, Iwata H, Yamanaka T, Sasano H, Toi M (2018) Impact of clinical response to neoadjuvant endocrine therapy on patient outcomes: a follow-up study of JFMC34-0601 multicentre prospective neoadjuvant endocrine trial. ESMO Open 3:e000314. https://doi.org/10.1136/esmoopen-2017-000314
Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, Moy B, Bardia A (2016) Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol 2:1477–1486. https://doi.org/10.1001/jamaoncol.2016.1897
Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ, Parmigiani G, Trippa L, Bardia A (2020) Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 26:2838–2848. https://doi.org/10.1158/1078-0432.CCR-19-3492
Criscitiello C, Disalvatore D, De Laurentiis M, Gelao L, Fumagalli L, Locatelli M, Bagnardi V, Rotmensz N, Esposito A, Minchella I, De Placido S, Santangelo M, Viale G, Goldhirsch A, Curigliano G (2014) High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer. Breast 23:69–75. https://doi.org/10.1016/j.breast.2013.11.007
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, Salter J, Detre S, Hills M, Walsh G, IMPACT Trialists Group (2007) Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167–170. https://doi.org/10.1093/jnci/djk020
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer J-U, Ashley SE, Francis S, Boeddinghaus I, Walsh G, IMPACT Trialists Group (2005) Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23:5108–5116. https://doi.org/10.1200/jco.2005.04.005
Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, Chaudri Ross HA, von Kameke A, Miller WR, Smith I, Eiermann W, Dowsett M (2008) Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 100:1380–1388. https://doi.org/10.1093/jnci/djn309
Ellis MJ, Suman VJ, Hoog J et al (2017) Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 35:1061–1069. https://doi.org/10.1200/jco.2016.69.4406
Kalinsky K, Barlow WE, Meric-Bernstam F et al (2020) SWOG S1007: first results from a phase III randomized clinical trial of standard adjuvant endocrine therapy +/- chemotherapy in patients with 1–3 positive nodes, hormone receptor-positive and HER2-negative breast cancer with recurrence scores ≤ 25. Presented at the 2020 San Antonio Breast Cancer Symposium (SABCS). December 8–11, 2020. Abstract GS3–00
Acknowledgements
The authors thank all the registered patients and their families, all the investigators and the Comprehensive Support Project for Oncology Research office in the NEOS trial, and Ms. Yumiko Nomura of Japan Clinical Research Support Unit for supporting the study. We acknowledge the support of ASCA Corporation in the editing of a draft of this manuscript.
Funding
This work was supported by the Comprehensive Support Project for Oncology Research of the Public Health Research Foundation (Japan). The funder supported the steering committee meeting and had a role in writing the report, but had no role in study design, data collection, data analysis, or data interpretation. The authors also thank AstraZeneca K.K., Bayer Yakuhin, Ltd., Bristol-Myers Squibb K.K., Chugai Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Sanofi K.K., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. for their generous donations.
Author information
Authors and Affiliations
Contributions
HI was the principal investigator, involved in all aspects of the study including conceptualization, development of the methodology, data curation, analysis, management, verification and interpretation, funding acquisition, project administration, supervision, and writing the first draft. YY, TS, YH, RN, HA, SO, MK, NT, TT, TF, and NM contributed to project administration. YY, SO, MK, NT, TT, and TF collected and interpreted the data. YS contributed to development of the methodology. HS contributed to development of the methodology and supervision. TY contributed to visualization, development of the methodology, data curation, verification, and statistical analysis. HI and TY had full access to all of the data, were involved in the writing, editing and critically reviewing the manuscript. All the authors have read and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
Hiroji Iwata: Consulting fees: Daichi Sankyo, Chugai, AstraZeneca, Sanofi, Lilly, MSD, Pfizer, and Novartis; Honoraria: Daiichi Sankyo, Chugai, AstraZeneca, Lilly, MSD, and Pfizer; Advisory Board: Daichi Sankyo, Chugai, AstraZeneca, Sanofi, Lilly, MSD, Pfizer, and Novartis. Yutaka Yamamoto: Research Funding: MSD Oncology, Chugai, Eisai, Daiichi Sankyo, Nippon Kayaku, Taiho, AstraZeneca, Takeda, and Pfizer; Honoraria: AstraZeneca, Chugai, Kyowa Kirin, Novartis, Lilly Japan, Pfizer, Daiichi Sankyo, MSD, Taiho, Eisai, Takeda, and Sysmex; Advisory Board: AstraZeneca, Chugai, Novartis, Lilly, Pfizer, Daiichi Sankyo, and MSD; Member of the Board of Directors: Japanese Breast Cancer Society and Japan Breast Cancer Research Group. Shoichiro Ohtani: Honoraria: Chugai, Lilly, Pfizer, AstraZeneca, and Eisai. Masahiro Kashiwaba: Honoraria: Chugai, Kyowa Kirin, Lilly Japan, Shionogi, Taiho, Daiichi Sankyo, Eisai, AstraZeneca, Pfizer, and Novartis. Naruto Taira: Honoraria: Pfizer, AstraZeneca, Eisai, ACTmed, and Kyowa Kirin. Tatsuya Toyama: Honoraria: Chugai, Eisai, Novartis, Takeda, Nippon Kayaku, AstraZeneca, Pfizer, and Lilly. Norikazu Masuda: Research Funding: Chugai, Eli Lilly, AstraZeneca, Pfizer, Daiichi Sankyo, MSD, Eisai, Novartis, Sanofi, Kyowa Kirin, and Nippon Kayaku; Honoraria: Eisai, Novartis, Sanofi, Kyowa Kirin, and Nippon Kayaku; Executive Board: Japan Breast Cancer Research Group; Board of Directors: Japanese Breast Cancer Society. Takuhiro Yamaguchi: Research Funding: Kyowa Kirin, AC Medical, A2 Healthcare, Facet Biotech, Japan Tobacco, Japan Media, Medidata Solutions, Ono, Intellim Corporation, Welby, 3H Medi Solution, Nipro, Hemp Kitchen, Nobori, Puravida Technologies, Medrio, 3H Clinical Trial, Senju Pharmaceutical, Otsuka, Eisai, and ClinChoice; Consulting or Advisory Role: EP Croit, Japan Tobacco, Ono, Kowa Company, Daiichi Sanko, Eisai, Chugai, 3H Clinical Trial, Sonire Therapeutics, Seikagaku Corporation, Otsuka, and Incyte Biosciences; Stock and Other Ownership Interests: STATCOM; Speakers' Bureau: Daiichi Sankyo, Takeda, Mebix, and AstraZeneca. Other authors have nothing to disclose.
Ethical approval
This study complied with the Declaration of Helsinki and the Ethical Guides to Clinical Investigations established by the Ministry of Health, Labour and Welfare. The study protocol and all other forms were approved by the ethics committee or internal review board at each study site.
Consent to participate
Signed informed consent was obtained from each participant before initiating any procedures.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwata, H., Yamamoto, Y., Sakai, T. et al. Phase III study of long-term prognosis of estrogen receptor-positive early breast cancer treated with neoadjuvant endocrine therapy with/without adjuvant chemotherapy. Breast Cancer Res Treat 199, 231–241 (2023). https://doi.org/10.1007/s10549-023-06874-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06874-7