Abstract
Aims
Clinicians use multi-gene/biomarker prognostic tests and free online tools to optimize treatment in early ER+/HER2− breast cancer. Here we report the comparison of recurrence risk predictions by CanAssist Breast (CAB), Nottingham Prognostic Index (NPI), and PREDICT along with the differences in the performance of these tests across Indian and European cohorts.
Methods
Current study used a retrospective cohort of 1474 patients from Europe, India, and USA. NPI risk groups were categorized into three prognostic groups, good (GPG-NPI index ≤ 3.4) moderate (MPG 3.41–5.4), and poor (PPG > 5.4). Patients with chemotherapy benefit of < 2% were low-risk and ≥ 2% high-risk by PREDICT. We assessed the agreement between the CAB and NPI/PREDICT risk groups by kappa coefficient.
Results
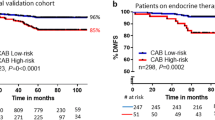
Risk proportions generated by all tools were: CAB low:high 74:26; NPI good:moderate:poor prognostic group- 38:55:7; PREDICT low:high 63:37. Overall, there was a fair agreement between CAB and NPI[κ = 0.31(0.278–0.346)]/PREDICT [κ = 0.398 (0.35–0.446)], with a concordance of 97%/88% between CAB and NPI/PREDICT low-risk categories. 65% of NPI-MPG patients were called low-risk by CAB. From PREDICT high-risk patients CAB segregated 51% as low-risk, thus preventing over-treatment in these patients. In cohorts (European) with a higher number of T1N0 patients, NPI/PREDICT segregated more as LR compared to CAB, suggesting that T1N0 patients with aggressive biology are missed out by online tools but not by the CAB.
Conclusion
Data shows the use of CAB in early breast cancer overall and specifically in NPI-MPG and PREDICT high-risk patients for making accurate decisions on chemotherapy use. CAB provided unbiased risk stratification across cohorts of various geographies with minimal impact by clinical parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Not all patients diagnosed with early-stage ER+/HER2 neu negative breast cancer are required to be treated with adjuvant chemotherapy [1]. Oncologists rely upon clinical and pathological parameters such as tumor size, node status, tumor grade, and menopausal status, to assess the risk of cancer recurrence and accordingly determine the need for chemotherapy [1, 2]. Over time, computational algorithms, and mathematical equations have been developed using the clinical outcome data of various cancer registries. [3, 4]. Nottingham prognostic index (NPI), Adjuvant, NHS PREDICT, IHC4, and CancerMath are the widely used freely available prognostic tools for ER+/HER2 neu negative early breast cancer patients [5,6,7,8,9,10].
NPI is an established prognostic tool developed in the UK in 1982 that uses three clinical parameters to risk categorize the patients [11]. NHS PREDICT, on the other hand, is an online tool used to project the overall survival and disease outcomes with/without adjuvant chemotherapy that is developed on a cohort of patients in the U.K [6]. These online tools based on clinicopathological parameters provide doctors with an exact percentage of absolute chemotherapy benefit or survival estimates for each patient which helps in explaining treatment strategy to the patient. While these tools are useful to clinicians as they are free, and available online thus helping to prescribe treatment to patients without any delay, there are certain limitations. These online tools have been reported to underestimate or overestimate survival in patients of certain age groups. In patients under 40 years, PREDICT overestimates all-cause mortality by 8% [2, 12, 13]. This data raises the question of universal adaptability of these online tools which primarily function based on clinical parameters, age and proliferation markers. Moreover, relying on online tools which do not consider the deeper biology of the disease with an appropriate statistical approach may deprive patients of adequate treatment, particularly in those with small but biologically aggressive tumors who may benefit from chemotherapy [2, 13]. For a heterogeneous disease like breast cancer, a prognostic test that looks at the biology of the disease in depth beyond anatomy, proliferation, and hormonal indices along with clinical parameters and coupled with an AI/ML-based algorithm will certainly give the test an edge of high accuracy [14].
CanAssist Breast (CAB), a prognostic test developed based on Indian patients’ tumor tissues tries to balance all the factors mentioned above. CAB is a proteomics-based test that analyses the expression of 5 protein biomarkers using immunohistochemistry technique. This information coupled with the patient’s tumor-specific information (tumor size, grade, and node status) is used by a machine learning-based algorithm to compute the risk of cancer recurrence [15]. CAB segregates patients into two actionable risk groups, i.e., ‘low or high’ risk for cancer recurrence. CAB has been validated on more than 3500 patients across India, USA, and Europe and has been used prospectively in ~ 3000 patients to date to plan treatment since its launch [16,17,18,19].
The aims of the current report are multi-fold. Here we (i) compare the recurrence risk predictions of CAB with online tools, NPI and PREDICT, (ii) identify the sub-groups where CAB risk predictions imply precise treatment plan and finally, (iii) compare the risk predictions by these tools across different geographies to assess the influence of racial and ethnic differences, if any.
Methods
Patient samples/Data collection
This retrospective study included formalin-fixed paraffin-embedded (FFPE) tumor tissues from 1474 early-stage (stage I, II, and IIIA) hormone receptor-positive, HER2/neu negative consecutive breast cancer patients (with follow-up available) collected from hospitals and biorepositories in India (n = 473), USA (n = 137), and Europe (n = 864) diagnosed between 2007 and 2016. Primary surgical blocks of FFPE tumor specimens from these patients along with patient age, clinical parameters, and treatment follow-up details were obtained from the respective treating hospital or biorepository. These samples were part of the earlier CAB validation studies [16,17,18]. The samples were obtained with the IRB and ethics committee approvals of all the participating hospitals.
CAB
Following haematoxylin & eosin staining to assess tumor quality, IHC was performed on consecutive sections for five CAB biomarkers (CD44, ABCC4, ABCC11, N-Cadherin, pan-Cadherin) on an automated Ventana platform [20, 21]. Post IHC grading by trained oncopathologists; this absolute percentage grading information for CD44, ABCC4, ABCC11, N-Cadherin, and pan-Cadherin, staining intensity for pan-Cadherin along with node status, tumor size, and tumor grade were used as inputs into the SVM—based algorithm to arrive at low-risk (CAB Risk Score ≤ 15.5) or high risk (CAB Risk Score > 15.5) category for each patient [15].
NPI
NPI uses the following equation of clinical parameters to arrive at an index.
NPI = maximum invasive tumor size (S in cm) × 0.2 + lymph node stage (LN = 1, 2, or 3) + histological grade (H = 1, 2, or 3).
The NPI index generated six categories [11]. In our study, excellent and good prognostic groups were merged and called “Good Prognostic Group” (NPI score ≤ 3.4); moderate I and II prognostic groups (NPI score > 3.41 to ≤ 5.4) were merged and called “Moderate Prognostic Group” and the poor prognostic group I and II (NPI score ≥ 5.4) were merged and called “Poor Prognostic Group”.
PREDICT
PREDICT V2 (https://breast.predict.nhs.uk/) is an online tool that predicts overall survival (OS) by providing a magnitude of benefit from chemotherapy over multiple time periods (5, 10, 15 years) using multiple parameters [22]. Based on the inputs selected, the tool gives a 5-year OS rate (%) including additional chemotherapy benefit (%). 2% (and above) difference in survival rates of patients treated with hormone therapy and 3rd generation chemotherapy was considered as chemotherapy benefit. In this analysis patients with < 2% chemotherapy benefit was considered as low-risk (LR) and ≥ 2% as high-risk (HR).
Statistical analysis
Rates of recurrence at a distant site along with SE (standard error) are computed from Kaplan–Meier survival curves. Cox proportional hazards model has been used to compute hazard ratios. Concordance between CAB, NPI, and PREDICT risk categories were analyzed by kappa coefficient [23]. For kappa correlation NPI moderate and high-risk groups were combined. The P-value was double-sided and < 0.05 was considered significant.
Results
Description of the cohort
The study cohort included 1474 patients (Table 1). Thirty-three percent of the cohort were aged below or equal to 50 years and 67% were above 50 years. The median age of the cohort was 57 years (range 26–92).
Fifty-four percent of the cohort had patients with T1 tumors and 45% had T2 tumors. Sixty-three percent of the cohort had node-negative tumors, 36.6% with N1 tumors, and 0.5% of the patients had N2 tumors. Sixty-three percent of the patients had G2 tumors, 23% were with poorly differentiated tumors (G3) and only 14% had G1 tumors. All patients were with ER-positive disease. Ki-67 data were available for 1159 patients, of which 66% of patients expressed Ki67 less than 14%. This cohort included patients from different geographical locations, Europe (59%), India (32%), and the USA (9%).
Risk proportions comparison of CAB, NPI, and PREDICT in the total cohort
CAB stratified 74% of the cohort as low-risk (LR) and 26% as high-risk (HR) (Table 2). NPI classified 38% of the patients as Good + excellent prognostic group-(GPG) and 55% of the patients as Moderate prognostic I and II (MPG) and 7% were poor prognostic-(PPG). Whereas PREDICT classified 63% of the cohort as LR and only 37% of the patients as HR. We observed similar risk stratification by CAB across age groups. Whereas, with NPI and PREDICT, GPG and LR proportions increased with the age of the patient. LR proportions decreased with all the prognostic tools with an increase in tumor size, increase in the number of nodes with metastasis, and higher grade; however the decrease was drastic for NPI and PREDICT. A tremendous drop was observed in NPI-GPG from 66.9% in T1 tumors to 3% in T2 tumors. Similarly, PREDICT showed a significant decrease in LR patients from 85% in T1 tumors to 37% in T2 tumors. However, with CAB the decrease in LR patients was not so drastic with 84% as LR in patients with T1 tumors and 64% in T2 tumors (Table 2). When we looked at the risk proportions in patients across grades, most of the patients with G1 tumors were called LR by all three tests. In G2 tumors, LR percentages were higher by CAB (83%) and PREDICT (72%) compared to NPI that identified only 41.6% as GPG. In patients with G3 tumors it is worthy to note CAB identified 40% as LR while PREDICT LR were 15%, whereas by NPI none of these patients were called GPG. It is notable to observe there were no PPG patients in N0, G1, and T1N0 sub-groups by NPI. It is interesting to see similar proportions of LR by CAB (91%) and PREDICT (92%) in the T1N0 sub-group although it was not the case in T1 and N0 sub-groups individually by each of these two tests, CAB and PREDICT (Table 2).
A similar trend of risk stratification is observed even in node-based sub-groups. Of the three tests, LR patients were higher by CAB (86%) and PREDICT (79%) compared to NPI (56%) in node-negative patients. In the N1 sub-group, LR patients were higher by CAB (56%) followed by PREDICT (35%) and they were very low by NPI (6%) (Table 2).
Distant event rates in various risk groups
The distant event rates within five years of breast cancer diagnosis remained less than 10% in the LR group/GPG of all three tests (Table 3). Although, the hazard ratio (HR) in the total cohort was significant by all tests, in the clinical high-risk sub-groups (T2/N1/G3 tumors) HR by at least one test or both tests (NPI and PREDICT) was not significant (Table 3). Unlike these two tests, CAB had a significant HR in all the sub-groups stratified by clinical parameters (Table 3).
Restratification of NPI and PREDICT risk groups by CAB
We observed a good agreement (97%) between CAB-LR and NPI-GPG in the total cohort. Only 3% of the NPI-GPG were called HR by CAB. 65% of the MPG and 35% PPG group were identified as LR by CAB. Even between PREDICT-LR and CAB-LR, a good agreement of 88% was observed in the total cohort. 12% of the PREDICT-LR were segregated as CAB HR and at the same time, 51% of the PREDICT HR were classified as LR by CAB. Good agreement (> 96%) was seen across all the age groups between CAB-LR and PREDICT LR/NPI-GPG. Across various clinical sub-groups, a substantial number of NPI-MPG, NPI-PPG, and PREDICT-HR were called LR by CAB including G3 tumors where all of them were PPG by NPI (Table 4).
Concordance of CAB risk with NPI and PREDICT
For the kappa correlation analysis, NPI-MPG and PPG were combined and considered high-risk. As observed by kappa correlation there was a fair agreement between CAB and NPI [0.31 (95% CI 0.278–0.376)] and with CAB and PREDICT [0.398 (95% CI 0.35–0.446)]. Moderate agreement was observed between CAB and PREDICT in sub-groups of patients aged above 50 years [0.429 (0.369–0.488)], 60 years [0.484 (0.408–0.560)], and N0 patient [0.406 (0.332–0.481)] sub-groups. However, a moderate agreement was observed between CAB and NPI only in a subgroup of patients with T1 tumors [0.478 (0.414–0.543)] (Table 5). Even across NPI and PREDICT there was only moderate agreement in the total cohort [0.529 (0.492–0.566)] (Additional Table 1).
Comparison of risk stratification of CAB, NPI, and PREDICT across geographies:
The demographics of Indian and European cohorts are presented independently in Additional Table 2. More than half of the Indian cohort had patients with T2 (76%) and N1 (52.4%) tumors. The European and USA cohorts were dominated by patients with T1 (69.6 and 67%) and N0 tumors (70.5 and 74%). The median tumor size in the European cohort was 1.6 cm (range 0.2–5.4), whereas it was 2.5 cm in the Indian cohort (range 0.9–8). In the Indian, European, and USA cohorts CAB LR proportions were 68%, 77%, and 77%, respectively. Whereas GGPs by NPI were 11% in Indian cohort but they were 50% and 55% in European and USA cohorts, respectively. Likewise, LR proportions by PREDICT were 35% in Indian and they were above 70% in European and USA cohorts (Table 6).
Across the sub-groups of these two diverse cohorts, European and Indian, risk proportions by CAB were similar or with fewer differences, while risk proportions by NPI/PREDICT differed greatly (Tables 7, 8). In the European sub-cohort with an increase in tumor size from T1 to T2, the CAB LR decreased from 85 to 61% (Table 7) and in the Indian sub-cohort, they almost remained constant at 82% (T1) and 83% (T2) (Table 8). Whereas with NPI, in the European and Indian sub-cohorts, NPI-GPG were very low, at 4% and 3% in the T2 sub-group while in the T1 sub-group they were tremendously higher at 85% (European) and 41% (Indian). A similar trend was observed with PREDICT with respect to LR proportions in T1 and T2 sub-groups across both cohorts (Tables 7, 8). Even across the other clinical parameters (node status and histological grade); increased low-risk proportions by NPI and PREDICT correlated with clinical risk parameters reflective of low clinical risk (e.g., N0 versus N1 or G1 versus G3 tumors) while CAB risk stratification seems to be independent of this.
Discussion
Prognostication in early breast cancer has evolved over time from the use of clinicopathological factors as standalone parameters for recurrence risk prediction to the recent multi-gene/biomarker tests/tools with or without the inclusion of clinical parameters to predict accurate prognosis. Online tools although having proved to be valuable, are known to overestimate/underestimate survival and chemotherapy benefit in patients of certain age groups and races [12, 24,25,26,27,28,29]. With this data, the use of these online tools appears to be limited to certain groups of patients. Moreover, breast cancer being a heterogeneous disease has different disease outcomes across different races and ethnicities [30,31,32,33]. Use of prognostic test/tool developed on one cohort might have variable performance on a cohort that’s racially and ethnically different from its development cohort. On the other hand, the use of multi-gene tests in all patients across the globe is not plausible due to their costs. At this juncture of online tools and expensive multi-gene tests, CAB serves as a cost-efficient proteomics-based test with performance comparable to that of these multi-gene tests (Oncotype DX) [34]. In the current report, we compared the risk stratification by NPI and PREDICT with that of CAB to identify the clinical risk groups where CAB risk predictions could be more useful for planning therapy to the patients.
A recurrence risk assessment by NPI and PREDICT is primarily driven by the clinical parameters whereas tumor biology plays a critical role in recurrence risk assessment by CAB. There was a huge decrease in low-risk proportions with an increase in the clinical risk (increased tumor size, lymph node metastasis, and higher histological grade) with NPI/PREDICT as observed by a few NPI-GPG patients in patients with N1 or G3 orT2 tumors. Similarly, none of the patients with G1/N0 tumors (lower clinical risk) and few patients with T1 and G2 tumors belonged to the NPI-PPG category. A similar pattern was observed with PREDICT. This high correlation observed between risk proportions and clinical parameters for NPI/PREDICT was not seen with CAB, although one could see a moderate decrease (T1-84%, T2-64%) in low-risk patients with an increase in clinical risk from T1 to T2 and similarly in node-positive versus node-negative tumors. This performance of CAB with minimal influence by clinical parameters is because CAB gives more importance to tumor biology than clinicopathological factors for recurrence risk prediction [16,17,18]. Moreover, in the European and USA cohorts dominated by patients with clinically low-risk features (T1/N0 tumors) NPI and PREDICT low-risk patients were tremendously high. Indian cohort was very different from these two cohorts with a higher representation of clinically high-risk patients (patients with T2/N1 tumors) and therefore had decreased NPI-GPG and PREDICT low-risk proportions compared to European and USA cohorts. However, even in these divergent cohorts, with respect to clinical parameters, CAB risk proportions across these three cohorts, did not vary to the extent as observed with NPI/PREDICT. We have previously shown that CAB’s accuracy in predicting distant recurrence in the European cohort is similar to that of the Indian cohort [18]. This is because CAB assesses the risk of recurrence by assessing the expression of the proteins (CD44, ABCC4, ABCC11, N-Cadherin, pan-Cadherin) involved in critical signaling pathways involved in the invasion of blood vessels by tumor cells leading to the spread, and drug resistance pathways; other than hormone signaling (ER, PR, and associated genes) and cell division/cell death (MDM2, MELK, PTTG1, etc. [35,36,37,38] and is thus less influenced by the clinical parameters and racial differences as well.
Another interesting group where this phenomenon of dependency of NPI/PREDICT on clinical parameters is well exhibited is the sub-group that had a mixture of clinical low- and high-risk features (T1N0 with histological grade 3). There were 89 T1N0 patients with grade 3 tumors. All these patients were called MPG by NPI. Although CAB and PREDICT identified similar numbers as low-risk in this sub-group (45 by CAB and 43 by PREDICT), there was only 48% concordance in these low-risk patients between CAB and PREDICT (k = 0.011 (95% CI, 0.19 to 0.219). This low concordance depicts LR patients by CAB and PREDICT are different. With respect to clinical outcomes CAB had a higher distant metastasis-free survival (DMFS) (95.6%) compared to PREDICT (90.7%) demonstrating that CAB low-risk predictions correlate with better survival outcomes than PREDICT (data not shown). Published data shows that the CAB ‘low risk’ patients with T1N0 disease have an excellent DMFS of 98% [17]. Though NPI, PREDICT, and CAB risk prediction models all have tumor size, node status, tumor grade as common parameters, the utmost important feature present additionally in CAB which is missing in NPI/PREDICT is the analysis of tumor biology. This data once again re-iterates the importance of tumor biology in accurate risk stratification in these patients. This is critical for patient care as it helps to prevent not only over-treatment of patients but at times under treatment of ‘clinically low-risk’ patients.
Concordance in the low-risk groups between two prognostic tests boosts the confidence of the clinician in avoiding chemotherapy. CAB has a very good concordance with multi-gene prognostic tests with 83% of concordance between CAB and Oncotype DX and MammaPrint in the low-risk category [18, 28]. Here in the current report, it is notable to observe 97% concordance between CAB and NPI low-risk categories where the NPI low-risk proportions were just half of the CAB low-risk proportions. While with PREDICT, a concordance of 88% was observed in the low-risk category indicating that CAB helps in identifying patients requiring chemotherapy of the remaining 12% of PREDICT low-risk patients; additionally prevents overtreatment in PREDICT high-risk patients as 51% of PREDICT high-risk patients were called as low-risk by CAB. However, in the NPI-MPG where treatment decision-making is ambiguous, 65% were stratified as low-risk by CAB which was higher than PREDICT that stratified only 45% as low-risk (Table 3), with similar survival advantage by both CAB and PREDICT risk stratifications (survival rate improved by 1.7 in NPI-MPG-CAB low-risk group and 1.8 in NPI-MPG-PREDICT low-risk group [data not shown]). Thus, the use of CAB in the NPI-MPG helps patients plan therapy accurately. The NICE diagnostic guidelines recommend the use of multi-gene tests (Oncotype DX, Prosigna, and EndoPredict) in NPI-MPG patients where chemotherapy decisions are unclear, and the use of these multi-gene tests is shown to be an expensive affair [39, 40]. The committee does not recommend the use of IHC4 as it lacks analytical validity. Unlike IHC4, CAB though an immunohistochemistry-based test has undergone rigorous analytical validation proving the test is robust [20] along with extensive clinical validation [16,17,18]. There are also other equations available, Maggee score that works using the immunohistochemical gradings of ER, PR, HER2, Ki67 and clinical parameters and are known to have good concordance with Oncotype DX RS [41]. Recently we completed CAB validation in a DUTCH sub-cohort of patients who participated in the TEAM trial, randomized for hormone therapy regimens [42] and the manuscript is under review.
The strengths of the manuscript are the large cohort size of stage I and stage II breast cancer patients and patient cohorts from varied geographies. Limitations of the study include the lack of demonstration of the accuracy of CAB risk predictions in comparison with online tools in known patient cohorts who underwent treatment only as per NPI or PREDICT risk assessments. With respect to CAB, the limitation is the lack of data on a prospective cohort randomized for chemotherapy.
In summary, we conclude that CAB finds a greater number of low-risk patients, who could be spared chemotherapy compared to NPI and PREDICT due to the deep analysis of tumor biology it involves, over the online tools. In the NPI prognostic group with a greater than 3.4 index, CAB offered more precise prognostication than PREDICT. Moreover, in patients where treatment decisions are based on PREDICT, CAB could reduce over-treatment in many high-risk patients by stratifying them as low risk. Thus, we believe use of CAB is complementary to risk assessment by online tools and will help to make improved decisions to provide optimum treatment to breast cancer patients.
Data availability
The data analyzed during the current study was not available publicly as it contains patient information. The data generated in the current work based on patient information will be available from the corresponding author on reasonable request.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet 365:1687–1717
Chen LL, Nolan ME, Silverstein MJ, Mihm MC Jr, Sober AJ, Tanabe KK et al (2009) The impact of primary tumor size, lymph node status, and other prognostic factors on the risk of cancer death. Cancer 115(21):5071–5083
Wazir U, Mokbel K, Carmichael A, Mokbel K (2017) Are online prediction tools a valid alternative to genomic profiling in the context of systemic treatment of ER-positive breast cancer? Cell Mol Biol Lett 22:20
Mokbel K, Wazir U, El Hage CH, Manson A, Choy C, Moye V et al (2017) A comparison of the performance of EndoPredict clinical and NHS PREDICT in 120 patients treated for ER-positive Breast Cancer. Anticancer Res 37(12):6863–6869
van Maaren MC, van Steenbeek CD, Pharoah PDP, Witteveen A, Sonke GS, Strobbe LJA et al (2017) Validation of the online prediction tool PREDICT v. 2.0 in the Dutch breast cancer population. Eur J Cancer 86:364–372
Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G et al (2010) PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 12(1):R1
Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD et al (2005) Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 23(12):2716–2725
Fong Y, Evans J, Brook D, Kenkre J, Jarvis P, Gower-Thomas K (2015) The Nottingham Prognostic Index: five- and ten-year data for all-cause survival within a screened population. Ann R Coll Surg Engl 97(2):137–139
Cuzick J, Dowsett M, Pineda S et al (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the genomic health recurrence score in early breast cancer. J Clin Oncol 29:4273–4278
Michaelson JS, Chen LL, Bush D, Fong A, Smith B, Younger J (2011) Improved web-based calculators for predicting breast carcinoma outcomes. Breast Cancer Res Treat 128(3):827–835
Haybittle JL, Blamey RW, Elston CW et al (1982) A prognostic index in primary breast cancer. Br J Cancer 45(3):361–366
Engelhardt EG, van den Broek AJ, Linn SC, Wishart GC, Rutgers EJT, van de Velde AO et al (2017) Accuracy of the online prognostication tools PREDICT and adjuvant! for early-stage breast cancer patients younger than 50 years. Eur J Cancer 78:37–44
Plakhins G, Irmejs A, Gardovskis A, Subatniece S, Liepniece-Karele I, Purkalne G et al (2013) Underestimated survival predictions of the prognostic tools Adjuvant! Online and PREDICT in BRCA1-associated breast cancer patients. Fam Cancer 12(4):683–689
Lou SJ, Hou MF, Chang HT, Chiu CC, Lee HH, Yeh SJ et al (2020) Machine learning algorithms to predict recurrence within 10 years after breast cancer surgery: a prospective cohort study. Cancers (Basel) 12(12):3817
Ramkumar C, Buturovic L, Malpani S, Kumar Attuluri A, Basavaraj C, Prakash C et al (2018) Development of a novel proteomic risk-classifier for prognostication of patients with early-stage hormone receptor-positive breast cancer. Biomark Insights 30(13):1177271918789100
Bakre MM, Ramkumar C, Attuluri AK, Basavaraj C, Prakash C, Buturovic L et al (2019) Clinical validation of an immunohistochemistry-based CanAssist-Breast test for distant recurrence prediction in hormone receptor-positive breast cancer patients. Cancer Med 8:1755–1764
Chandra Doval D, Mehta A, Somashekhar SP, Gunda A, Singh G, Bal A, Khare S et al (2021) The usefulness of CanAssist breast in the assessment of recurrence risk in patients of ethnic Indian origin. Breast 59:1–7
Gunda A, Basavaraj C, Chandra Prakash SV, Adinarayan M, Kolli R, Eshwaraiah MS, Saura C et al (2022) A retrospective validation of CanAssist Breast in European early-stage breast cancer patient cohort. Breast 63:1–8
Sankaran S, Dikshit JB, Prakash Sv C, Mallikarjuna SE, Somashekhar SP, Patil S et al (2021) CanAssist breast impacting clinical treatment decisions in early-stage HR+ breast cancer patients: Indian Scenario. Indian J Surg Oncol 12:21–29
Attuluri AK, Serkad CP, Gunda A, Ramkumar C, Basavaraj C, Buturovic L et al (2019) Analytical validation of CanAssist-Breast: an immunohistochemistry based prognostic test for hormone receptor positive breast cancer patients. BMC Cancer 19:1–10
Chandra Prakash SV, Attuluri AK, Basavaraj C, Adinarayan M, Krishnamoorthy N, Savitha BA et al (2021) Validation of CanAssist breast immunohistochemistry biomarkers on an automated platform and its applicability in tissue microarray. Int J Clin Exp Pathol 14:1013–1028
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22:276–282
Campbell HE, Taylor MA, Harris AL, Gray AM (2009) An investigation into the performance of the Adjuvant! Online prognostic programme in early breast cancer for a cohort of patients in the United Kingdom. Br J Cancer 101:1074–1084
de Glas NA, Bastiaannet E, Engels CC, de Craen AJM, Putter H, van de Velde CJH et al (2016) Validity of the online PREDICT tool in older patients with breast cancer: a population-based study. Br J Cancer 114:395–400
Mook S, Schmidt MK, Rutgers EJ, van de Velde AO, Visser O, Rutgers SM et al (2009) Calibration and discriminatory accuracy of prognosis calculation for breast cancer with the online Adjuvant! program: a hospital-based retrospective cohort study. Lancet Oncol 10:1070–1076
Lambertini M, Pinto AC, Ameye L, Jongen L, Mastro LD, Puglisi F et al (2016) The prognostic performance of Adjuvant! Online and Nottingham Prognostic Index in young breast cancer patients. Br J Cancer 115:1471–1478
Wong HS, Subramaniam S, Alias Z, Taib NA, Ho GF, Yip CH et al (2015) The predictive accuracy of PREDICT: a personalized decision-making tool for Southeast Asian women with breast cancer. Medicine (Baltimore) 94:e593
Maishman T, Copson E, Stanton L, Gerty S, Dicks E, Durcan L et al (2015) An evaluation of the prognostic model PREDICT using the POSH cohort of women aged_40 years at breast cancer diagnosis. Br J Cancer 112:983e91
Leong SP, Shen ZZ, Liu TJ et al (2010) Is breast cancer the same disease in Asian and Western countries? World J Surg 34:2308–2324
Lao C, Lawrenson R, Edwards M, Campbell I (2019) Treatment and survival of Asian women diagnosed with breast cancer in New Zealand. Breast Cancer Res Treat 177:497–505
Farooq S, Coleman MP (2005) Breast cancer survival in South Asian women in England and Wales. J Epidemiol Community Health 59:402–406
Iqbal J, Ginsburg O, Rochon PA et al (2015) Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 313:165–173
Sengupta AK, Gunda A, Malpani S, Serkad CPV, Basavaraj C, Bapat A et al (2020) Comparison of breast cancer prognostic tests CanAssist Breast and Oncotype DX. Cancer Med 21:7810–7818
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart A, Voskuil DW, Schreiber GJ et al (2002) A gene-expression signature a predictor of survival in breast cancer. N Engl J Med 347:1999–2009
Chia SK, Bramwell VH, Tu D, Shepherd LE, Jiang S, Vickery T et al (2012) A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res 18:4465–4472
Filipits M, Rudas M, Jakesz R, Dubsky P, Fitzal F, Singer CF et al (2011) A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 17:6012–6020
(2018) Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer Diagnostics guidance. www.nice.org.uk/guidance/dg34
Ward S, Scope A, Rafia R, Pandor A, Harnan S, Evans P et al (2013) Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review and cost-effectiveness analysis. Health Technol Assess. https://doi.org/10.3310/hta17440
Klein ME, Dabbs DJ, Shuai Y, Brufsky AM, Jankowitz R, Puhalla SL et al (2013) Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol 26:658–664
Zhang X, Gunda A, Meershoek-Klein-Kranenbarg E, Kuppen PJK, Savitha BA, Prakash C et al (2022) 9P-Long term recurrence risk predictions by CanAssist Breast in a sub-cohort of TEAM. ESMO Breast Cancer, Berlin
Acknowledgements
We acknowledge Dr. Savitha BA, Dr.Payal Srivastava , Dr. Nirupama Naidu, Dr. Naveen Krishnamoorthy, Dr. Manjula Aadinarayan, Chandra Prakash SV, Ramu Kolli for assisting us with histopathology work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AG and MB have conceived the idea of the manuscript. Data compilation was done by AG, Kiran and TK. Data analysis was done by AG. Manuscript was drafted by AG, MSE, Kiran and TK, reviewed by AG and MB. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interest
There is no conflict of interest. All authors are employees of OncoStem Diagnostics Pvt Ltd.
Ethical approval and consent to participate
Clearance for the samples used in this study has been obtained from the Bangalore ethical committee (ECR/87/Indt/KA/2013) and in accordance with the Declaration of Helsinki. The experimental protocols have been approved the IRB (Institutional Review Board) and Ethics committees of all the participating hospitals/centers. The study being retrospective in nature patient consent was waived off as per the ICMR (Indian Council of Medical Research) guidelines regarding the informed consent for studies involving retrospective, non-interventional anonymized biological samples/data.
Consent for publication
Is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunda, A., Eshwaraiah, M.S., Gangappa, K. et al. A comparative analysis of recurrence risk predictions in ER+/HER2− early breast cancer using NHS Nottingham Prognostic Index, PREDICT, and CanAssist Breast. Breast Cancer Res Treat 196, 299–310 (2022). https://doi.org/10.1007/s10549-022-06729-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06729-7