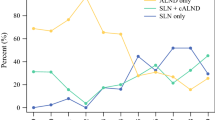
Abstract
Purpose
This study aimed to evaluate whether axillary lymph node dissection (ALND) can be omitted in patients with 1–2 positive sentinel lymph nodes (SLNs) who received total mastectomy (TM).
Methods
Consecutive breast cancer patients with 1–2 positive SLNs were retrospectively reviewed from a multi-institutional database. Patients were divided into sentinel lymph node biopsy (SLNB) group and ALND group. Administration of adjuvant chemotherapy and survival were compared between groups. To further verify the results, a meta-analysis was also conducted.
Results
Among the 1161 enrolled patients, 893 (76.9%) received ALND and 268 (23.1%) underwent SLNB alone. Administration of chemotherapy was comparable between the two groups (91.1% vs. 90.6%, P = 0.798), which was consistent in TM (P = 0.638) and BCS cohort (P = 0.576). After a median follow-up of 36 months, no significant difference was observed between the two groups in recurrence-free survival (P = 0.583) regardless of surgery of breast. During further meta-analysis, 13 out of 4733 relative studies reported the association of axillary surgery and disease-free survival (DFS) or overall survival (OS) in 1–2 positive SLNs patients. Pooled analysis showed no difference in adjusted DFS (HR 0.84, 95% CI 0.70–1.02) or OS (HR 1.02, 95% CI 0.93–1.11) between SLNB and ALND groups. Survival benefit of ALND remained non-significant after restricting the analysis in four studies with patients only receiving BCS, or in three studies with patients only receiving TM.
Conclusion
Further ALND does not impact adjuvant chemotherapy administration or disease outcome in breast cancer patients with 1–2 positive SLNs treated with TM.





Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALND:
-
Axillary lymph node dissection
- SLNB:
-
Sentinel lymph node biopsy
- SLN:
-
Sentinel lymph node
- ACOSOG:
-
American College of Surgeons Oncology Group
- BCS:
-
Breast-conserving surgery
- TM:
-
Total mastectomy
- SJTU-BCDB:
-
Shanghai Jiao Tong University-Breast Cancer Database
- DCIS:
-
Ductal carcinoma in situ
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular cancer
- non-SLN:
-
non-sentinel lymph node
- RFS:
-
Recurrence-free survival
- ACT:
-
Adjuvant chemotherapy
- HR:
-
Hazard ratio
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER-2:
-
Human epidermal growth factor receptor-2
- SLNR:
-
Sentinel lymph node ratio
- OR:
-
Odds ratio
- LRR:
-
Local–regional recurrence
References
Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, Colleoni M, Denkert C, Piccart-Gebhart M, Regan M, Senn HJ, Winer EP, Thurlimann B (2019) Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen international consensus guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 30:1541–1557. https://doi.org/10.1093/annonc/mdz235
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575. https://doi.org/10.1001/jama.2011.90
Dengel LT, Van Zee KJ, King TA, Stempel M, Cody HS, El-Tamer M, Gemignani ML, Sclafani LM, Sacchini VS, Heerdt AS, Plitas G, Junqueira M, Capko D, Patil S, Morrow M (2014) Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol 21:22–27. https://doi.org/10.1245/s10434-013-3200-6
Gao W, Zeng Y, Fei X, Chen X, Shen K (2020) Axillary lymph node and non-sentinel lymph node metastasis among the ACOSOG Z0011 eligible breast cancer patients with invasive ductal, invasive lobular, or other histological special types: a multi-institutional retrospective analysis. Breast Cancer Res Treat 184:193–202. https://doi.org/10.1007/s10549-020-05842-9
Yi M, Giordano SH, Meric-Bernstam F, Mittendorf EA, Kuerer HM, Hwang RF, Bedrosian I, Rourke L, Hunt KK (2010) Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. https://doi.org/10.1245/s10434-010-1253-3
Yi M, Kuerer HM, Mittendorf EA, Hwang RF, Caudle AS, Bedrosian I, Meric-Bernstam F, Wagner JL, Hunt KK (2013) Impact of the american college of surgeons oncology group Z0011 criteria applied to a contemporary patient population. J Am Coll Surg 216:105–113. https://doi.org/10.1016/j.jamcollsurg.2012.09.005
Wang J, Mittendorf EA, Sahin AA, Yi M, Caudle A, Hunt KK, Wu Y (2014) Outcomes of sentinel lymph node dissection alone vs. axillary lymph node dissection in early stage invasive lobular carcinoma: a retrospective study of the surveillance, epidemiology and end results (SEER) database. PLoS ONE 9:e89778–e89778. https://doi.org/10.1371/journal.pone.0089778
Lee J, Choi JE, Kim SJ, Lee SB, Seong MK, Jeong J, Yoon CS, Kim BK, Sun WY (2018) Comparative study between sentinel lymph node biopsy and axillary dissection in patients with one or two lymph node metastases. J Breast Cancer 21:306–314. https://doi.org/10.4048/jbc.2018.21.e44
Jung J, Han W, Lee ES, Jung SY, Han JH, Noh DY, Kim Y, Choi HJ, Lee JE, Nam SJ, Lee JW, Kim HJ, Um E, Kim JH, Park S, Cho YU (2019) Retrospectively validating the results of the ACOSOG Z0011 trial in a large Asian Z0011-eligible cohort. Breast Cancer Res Treat 175:203–215. https://doi.org/10.1007/s10549-019-05157-4
Bilimoria KY, Bentrem DJ, Hansen NM, Bethke KP, Rademaker AW, Ko CY, Winchester DP, Winchester DJ (2009) Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol 27:2946–2953. https://doi.org/10.1200/jco.2008.19.5750
Solá M, Alberro JA, Fraile M, Santesteban P, Ramos M, Fabregas R, Moral A, Ballester B, Vidal S (2013) Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol 20:120–127. https://doi.org/10.1245/s10434-012-2569-y
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol 14:297–305. https://doi.org/10.1016/s1470-2045(13)70035-4
Park HS, Chae BJ, Song BJ, Jung SS, Han W, Nam SJ, Youn HJ, Ko BK, Kim DW (2014) Effect of axillary lymph node dissection after sentinel lymph node biopsy on overall survival in patients with T1 or T2 node-positive breast cancer: report from the Korean Breast Cancer Society. Ann Surg Oncol 21:1231–1236. https://doi.org/10.1245/s10434-013-3350-6
Fu Y, Chung D, Cao MA, Apple S, Chang H (2014) Is axillary lymph node dissection necessary after sentinel lymph node biopsy in patients with mastectomy and pathological N1 breast cancer? Ann Surg Oncol 21:4109–4123. https://doi.org/10.1245/s10434-014-3814-3
Kim BK, Park BW, Hur MH, Lee HB, Park MH, Jeong J, Lee HJ, Lee J, Kim D, Sun WY (2020) Omission of axillary lymph node dissection in patients who underwent total mastectomy with 1 or 2 metastatic lymph nodes. Ann surg treat res 98:283–290. https://doi.org/10.4174/astr.2020.98.6.283
Joo JH, Kim SS, Son BH, Ahn SD, Jung JH, Choi EK, Ahn SH, Lee JW, Kim HJ, Ko BS (2019) Axillary lymph node dissection Does not improve post-mastectomy overall or disease-free survival among breast cancer patients with 1–3 positive nodes. Cancer res treat : off j Korean Cancer Assoc 51:1011–1021. https://doi.org/10.4143/crt.2018.438
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE (2017) Sentinel lymph node biopsy for patients with early-stage breast cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol 35:561–564. https://doi.org/10.1200/jco.2016.71.0947
Tseng J, Alban RF, Siegel E, Chung A, Giuliano AE, Amersi FF (2021) Changes in utilization of axillary dissection in women with invasive breast cancer and sentinel node metastasis after the ACOSOG Z0011 trial. Breast J 27:216–221. https://doi.org/10.1111/tbj.14191
Poodt IGM, Spronk PER, Vugts G, van Dalen T, Peeters M, Rots ML, Kuijer A, Nieuwenhuijzen GAP, Schipper RJ (2018) Trends on axillary surgery in nondistant metastatic breast cancer patients treated between 2011 and 2015: a dutch population-based study in the ACOSOG-Z0011 and AMAROS era. Ann Surg 268:1084–1090. https://doi.org/10.1097/sla.0000000000002440
Tadros AB, Moo TA, Stempel M, Zabor EC, Khan AJ, Morrow M (2020) Axillary management for young women with breast cancer varies between patients electing breast-conservation therapy or mastectomy. Breast Cancer Res Treat 180:197–205. https://doi.org/10.1007/s10549-019-05520-5
Rudenstam CM, Zahrieh D, Forbes JF, Crivellari D, Holmberg SB, Rey P, Dent D, Campbell I, Bernhard J, Price KN, Castiglione-Gertsch M, Goldhirsch A, Gelber RD, Coates AS (2006) Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of international breast cancer study group trial 10–93. J Clin Oncol 24:337–344. https://doi.org/10.1200/jco.2005.01.5784
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 318:918–926. https://doi.org/10.1001/jama.2017.11470
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K (2010) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. https://doi.org/10.1097/SLA.0b013e3181f08f32
FitzSullivan E, Bassett RL, Kuerer HM, Mittendorf EA, Yi M, Hunt KK, Babiera GV, Caudle AS, Black DM, Bedrosian I, Reyna C, Teshome M, Meric-Bernstam F, Hwang R (2017) Outcomes of sentinel lymph node-positive breast cancer patients treated with mastectomy without axillary therapy. Ann Surg Oncol 24:652–659. https://doi.org/10.1245/s10434-016-5605-5
Milgrom S, Cody H, Tan L, Morrow M, Pesce C, Setton J, Rogers K, Arnold B, Eaton A, Catalano J, McCormick B, Powell S, Ho A (2012) Characteristics and outcomes of sentinel node-positive breast cancer patients after total mastectomy without axillary-specific treatment. Ann Surg Oncol 19:3762–3770. https://doi.org/10.1245/s10434-012-2386-3
Peristeri DV, Harissis HV (2021) Axillary lymph node dissection vs sentinel biopsy only among women with early-stage breast cancer and sentinel node metastasis: a systematic review and meta-analysis. Breast J 27:158–164. https://doi.org/10.1111/tbj.14140
Picado O, Khazeni K, Allen C, Yakoub D, Avisar E, Kesmodel SB (2018) Extent of regional lymph node surgery and impact on outcomes in patients with early-stage breast cancer and limited axillary disease undergoing mastectomy. Breast Cancer Res Treat 171:461–469. https://doi.org/10.1007/s10549-018-4840-9
Goyal A, Dodwell D (2015) POSNOC: a randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. Clin Oncol (R Coll Radiol) 27:692–695. https://doi.org/10.1016/j.clon.2015.07.005
de Boniface J, Frisell J, Andersson Y, Bergkvist L, Ahlgren J, Rydén L, OlofssonBagge R, Sund M, Johansson H, Lundstedt D (2017) Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer 17:379. https://doi.org/10.1186/s12885-017-3361-y
Funding
This work was supported by the National Natural Science Foundation of China (No. 81772797); Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (No. 20172007); and Ruijin Hospital, Shanghai Jiao Tong University School of Medicine—“Guangci Excellent Youth Training Program” (No. GCQN-2017-A18). The funders had no role in study design, data collection, data analysis, article writing, or article submission.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by WG, SL, XC and YZ. The first draft of the manuscript was written by WG, SL and YZ, and XC and KS revised the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no conflict of interest.
Ethical approval
Current study was reviewed and approved by independent ethical committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, W., Lu, S., Zeng, Y. et al. Axilla lymph node dissection can be safely omitted in patients with 1–2 positive sentinel nodes receiving mastectomy: a large multi-institutional study and a systemic meta-analysis. Breast Cancer Res Treat 196, 129–141 (2022). https://doi.org/10.1007/s10549-022-06727-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06727-9