Abstract
Purpose
Genomic profiling in early-stage breast cancer provides prognostic and predictive information. Genomic profiling assays have not been validated in locally advanced breast cancer (LABC). We examined a large cancer registry to evaluate genomic profiling in LABC and its effect on treatment decisions and survival.
Methods
Females with ER+/HER2− LABC who did not receive neoadjuvant therapy were selected from the National Cancer Database 2004–2017. We compared characteristics between patients with and without genomic profiling and with low genomic risk, 21-gene recurrence score ≤ 25 or low-risk 70-gene signature, treated with endocrine therapy ± chemotherapy. Propensity score methods were utilized to account for covariates that may have predicted treatment. Univariable and multivariable survival analyses were performed.
Results
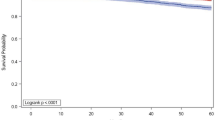
Of 18,437 patients with LABC, 1258 (7%) had genomic profiling and 1022 (81%) had low genomic risk results. 562 patients (55%) with low genomic risk received chemotherapy and endocrine therapy (chemoendocrine). Patients who received chemoendocrine therapy were younger, had fewer comorbidities, presented with higher stage disease, had higher grade tumors, more frequently had partial mastectomy, and more often received radiation than those who received endocrine therapy alone. On multivariable analysis, endocrine therapy alone was associated with worse OS compared to chemoendocrine therapy (HR 1.77, 95% CI 1.13–2.78, p = 0.013).
Conclusion
In women with LABC and low genomic risk, endocrine therapy alone was associated with worse OS compared to chemoendocrine therapy. This suggests that genomic profiling is not predictive in LABC. Accordingly, genomic profiling should not be routinely utilized to make adjuvant treatment decisions in LABC in the absence of further data which shows a benefit.



Similar content being viewed by others
References
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65
Cardoso F, Van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S et al (2016) 70--Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375(8):717–729
Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF et al (2021) 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 385(25):2336–2347
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121
Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22
Parsons BM, Landercasper J, Smith AL, Go RS, Borgert AJ, Dietrich LL (2016) 21-Gene recurrence score decreases receipt of chemotherapy in ER+ early-stage breast cancer: an analysis of the NCDB 2010–2013. Breast Cancer Res Treat 159(2):315–326
Kurian AW, Bondarenko I, Jagsi R, Friese CR, McLeod MC, Hawley ST et al (2018) Recent trends in chemotherapy use and oncologists’ treatment recommendations for early-stage breast cancer. J Natl Cancer Inst 110(5):493–500
Soliman H, Shah V, Srkalovic G, Mahtani R, Levine E, Mavromatis B et al (2020) MammaPrint guides treatment decisions in breast cancer: results of the IMPACt trial. BMC Cancer 20(1):81
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347(25):1999–2009
Buyse M, Loi S, van’t Veer L, Viale G, Delorenzi M, Glas AM et al (2006) Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst 98(17):1183–1192
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46(3):399–424
Kozick Z, Hashmi A, Dove J, Hunsinger M, Arora T, Wild J et al (2018) Disparities in compliance with the oncotype DX breast cancer test in the United States: a National Cancer Data Base assessment. Am J Surg 215(4):686–692
Davis BA, Aminawung JA, Abu-Khalaf MM, Evans SB, Su K, Mehta R et al (2017) Racial and ethnic disparities in oncotype DX test receipt in a statewide population-based study. J Natl Compr Cancer Netw 15(3):346–354
Press DJ, Ibraheem A, Dolan ME, Goss KH, Conzen S, Huo D (2018) Racial disparities in omission of oncotype DX but no racial disparities in chemotherapy receipt following completed oncotype DX test results. Breast Cancer Res Treat 168(1):207–220
Orucevic A, Heidel RE, Bell JL (2016) Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: lessons learned from the 2010 to 2012 National Cancer Data Base analysis. Breast Cancer Res Treat 157(3):427–435
Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA (2013) Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract 9(4):182–187
Piccart M, van’t Veer LJ, Poncet C, Lopes Cardozo JMN, Delaloge S, Pierga JY et al (2021) 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol 22(4):476–488
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J et al (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28(11):1829–1834
Gluz O, Nitz UA, Christgen M, Kates RE, Shak S, Clemens M et al (2016) West German Study Group Phase III PlanB Trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol 34(20):2341–2349
Nitz U, Gluz O, Christgen M, Kates RE, Clemens M, Malter W et al (2017) Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat 165(3):573–583
Saghatchian M, Mook S, Pruneri G, Viale G, Glas AM, Guerin S et al (2013) Additional prognostic value of the 70-gene signature (MammaPrint((R))) among breast cancer patients with 4–9 positive lymph nodes. Breast 22(5):682–690
Stemmer SM, Steiner M, Rizel S, Geffen DB, Nisenbaum B, Peretz T et al (2017) Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer 3:32
Ibraheem AF, Press DJ, Olopade OI, Huo D (2019) Community clinical practice patterns and mortality in patients with intermediate oncotype DX recurrence scores: who benefits from chemotherapy? Cancer 125(2):213–222
Weiser R, Haque W, Polychronopoulou E, Hatch SS, Kuo YF, Gradishar WJ et al (2021) The 21-gene recurrence score in node-positive, hormone receptor-positive, HER2-negative breast cancer: a cautionary tale from an NCDB analysis. Breast Cancer Res Treat 185(3):667–676
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30(15):1796–1804
Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC et al (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379(9814):432–444
Author information
Authors and Affiliations
Contributions
OP, RM, FV and SK contributed for study conception and design; OP, DK, and SK analyzed the data. OP and SK prepared the manuscript. KR, JC, LW, RM, FV, NG, and SK provided critical revisions to the manuscript. OP, DK, KR, JC, LW, RM, FV, NG, and SK reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Reshma Mahtani, D.O.: Consultant/Advisor: Agendia, Amgen, Astra Zeneca, Biotheranostics, Daiichi, Eisai, Genentech, Immuomedics, Lilly, Merck, Novartis, Pfizer, Puma, Sanofi, SeaGen. Kristin Rojas, M.D.: Research Funding, Bristol Myers Squibb Foundation; Speakers Honoraria, Pacira Pharmaceuticals and Roche; Consultant/Advisor, Clue App (Biowink) and Roche Diagnostic Solutions. Neha Goel, M.D.: Research Funding, NIH/NCI Funding K12CA226330. All other authors have no conflict of interest or disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Picado, O., Kwon, D., Rojas, K. et al. Impact of genomic assays on treatment and outcomes in locally advanced breast cancer. Breast Cancer Res Treat 194, 433–447 (2022). https://doi.org/10.1007/s10549-022-06625-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06625-0