Abstract
Purpose
To examine racial/ethnic disparities in Oncotype DX (ODX) testing among patients with node-negative, estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancers and possible racial/ethnic disparities in chemotherapy receipt following ODX testing within Recurrence Score (RS) category (Not Done, Low, Intermediate, High), as well as chemotherapy receipt time trends within RS categories.
Methods
A retrospective cohort list of 125,288 women who were potentially indicated for ODX testing from 2010 to 2014 was obtained using the National Cancer Database. We fit multivariate logistic regression predicting chemotherapy receipt, adjusting for clinical factors, patient demographic factors, and hospital-level factors, separately by RS category, and calculated odds ratios (OR) and 95% confidence intervals (CI), as well as time trends.
Results
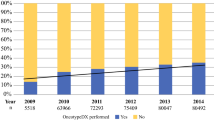
Overall, ODX testing was completed for 46.1% of Non-Hispanic (NH) Whites, 43.9% of NH Blacks, and 41.7% of Hispanics. Among patients who did not receive ODX testing, NH Black and Hispanic women both experienced statistically significant increases in chemotherapy receipt relative to NH White women (NH Black OR 1.23; 95% CI 1.11–1.37; Hispanic OR 1.23; 95% CI 1.07–1.42). However, among patients with ODX results, no statistically significant racial/ethnic differences in chemotherapy receipt were observed within strata of RS category. Trend analyses demonstrated increasing adherence to national guidelines for ODX testing.
Conclusions
We identified racial disparities in omission of ODX testing but no differences in chemotherapy receipt if ODX test results were obtained, suggesting increasing access to ODX testing may improve racial equality in efficacious use of adjuvant chemotherapy for ER-positive HER2-negative breast cancer.


Similar content being viewed by others
Abbreviations
- AJCC:
-
American joint committee on cancer
- ASCO:
-
American society of clinical oncology
- CMS:
-
Centers for medicare & medicaid services
- CCI:
-
Charlson/deyo Comorbidity Index
- CoC:
-
Commission on cancer
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- HR:
-
Hormone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- ICD-O-3:
-
International classification of diseases for oncology—third edition
- NCDB:
-
National cancer database
- NCI:
-
National cancer institute
- NCCN:
-
National comprehensive cancer network
- NH:
-
Non-Hispanic
- ODX:
-
Oncotype dx™
- PI:
-
Pacific islander
- POC:
-
Patterns of care
- RS:
-
Recurrence score
References
Carlson JJ, Roth JA (2013) The impact of the oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22
Hornberger J, Chien R, Krebs K, Hochheiser L (2011) US insurance program’s experience with a multigene assay for early-stage breast cancer. J Oncol Pract 7(3S):e38s–e45s
Kaelin WG (2005) The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer 5(9):689–698
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New Engl J Med 351(27):2817–2826
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312
National: Comprehensive Cancer Network (2012) NCCN clinical practice guidelines in oncology: breast cancer V.1.2015
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New Engl J Med 351(27):2817–2826
Roberts MC, Bryson A, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes K, Wheeler SB (2016) Oncologists’ barriers and facilitators for oncotype dx use: qualitative study. Int J Technol Assessment HealthC 32(5):355–361
Orucevic A, Heidel R, Bell J (2016) Abstract P3-07-17: Analysis of the National cancer data base 2010-2012 oncotype DX breast cancer assay: Lessons learned. Cancer Research. https://doi.org/10.1158/1538-7445.SABCS15-P3-07-17
Shak S, Petkov V, Miller D, Howlader N, Gliner N, Howe W, Schussler N, Cronin K, Baehner F, Penberthy L (2016) Abstract P5-15-01: Breast cancer specific survival in 38,568 patients with node negative hormone receptor positive invasive breast cancer and oncotype DX recurrence score results in the SEER database. Cancer Research. https://doi.org/10.1158/1538-7445.SABCS15-P5-15-01
Jasem J, Amini A, Rabinovitch R, Borges VF, Elias A, Fisher CM, Kabos P (2016) 21-Gene recurrence score assay as a predictor of adjuvant chemotherapy administration for early-stage breast cancer: an analysis of use, therapeutic implications, and disparity profile. J Clin Oncol 34(17):1995–2002
Parsons BM, Landercasper J, Smith AL, Go RS, Borgert AJ, Dietrich LL (2016) 21-Gene recurrence score decreases receipt of chemotherapy in ER + early-stage breast cancer: an analysis of the NCDB 2010–2013. Breast Cancer Res Treat 159(2):315–326
Orucevic A, Heidel RE, Bell JL (2016) Utilization and impact of 21-gene recurrence score assay for breast cancer in clinical practice across the United States: lessons learned from the 2010 to 2012 National cancer data base analysis. Breast Cancer Res Treat 157(3):427–435
DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, Jemal A (2016) Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA 66(4):290–308
Hirschman J, Whitman S, Ansell D (2007) The black:white disparity in breast cancer mortality: the example of Chicago. Cancer Cause Contr 18(3):323–333
Polite BN, Adams-Campbell LL, Brawley OW, Bickell N, Carethers JM, Flowers CR, Foti M, Gomez SL, Griggs JJ, Lathan CS (2017) Charting the Future of Cancer Health Disparities Research: A Position Statement From the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. J Clin Oncol. https://doi.org/10.1200/JCO.2017.73.6546
Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY (2009) Comparison of commission on cancer-approved and –nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol 27(25):4177–4181
Bilimoria KY, Stewart AK, Winchester DP, Ko CY (2008) The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 15(3):683–690
Bieging KT, Attardi LD (2012) Deconstructing p53 transcriptional networks in tumor suppression. Trends Cell Biol 22(2):97–106
Press DJ, Pharoah P (2010) Risk Factors for Breast Cancer: a reanalysis of two case-control studies from 1926 and 1931. Epidemiol 21(4):566–572
Gomez SL, Glaser SL (2006) Misclassification of race/ethnicity in a population-based cancer registry. Cancer Cause Control 17:771–781
Gomez SL, Le GM, West DW, Satariano WA, O’Connor L (2003) Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. Am J Public Health 93(10):1685–1688
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 40(5):373–383
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619
International Classification of Diseases for Oncology Third Edition (2013) First Revision. World Health Organization, Geneva
National Cancer Database. Charlson/Deyo Score. Retrieved from: http://ncdbpuf.facs.org/content/charlsondeyo-comorbidity-index
United States department of agriculture economic research service. rural-urban continuum codes. retrieved from: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes
National Cancer Database. income 2008–2012. retrieved from: http://ncdbpuf.facs.org/node/384
Cress RD, Chen YS, Morris CR, Chew H, Kizer KW (2016) Underutilization of gene expression profiling for early-stage breast cancer in California. Cancer Cause Control 27(6):721–727
Enewold L, Geiger AM, Zujewski J, Harlan LC (2015) Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat 151(1):149–156
Carlson R, Allred D, Anderson B (2009) NCCN clinical practice guidelines in oncology: breast cancer, v. 1.2009. National comprehensive cancer network, Washington
Oestreicher N, Ramsey SD, Linden HM, McCune JS, van’t Veer LJ, Burke W, Veenstra DL (2005) Gene expression profiling and breast cancer care: what are the potential benefits and policy implications? Genet Med 7(6):380–389
Kittaneh M, Montero AJ, Glück S (2013) Molecular profiling for breast cancer: a comprehensive review. Biomarkers Cancer 5:61
Marrone M, Stewart A, Dotson WD (2015) Clinical utility of gene-expression profiling in women with early breast cancer: an overview of systematic reviews. Genet Med 17(7):519
McVeigh TP, Kerin MJ (2017) Clinical use of the oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer 9:393
Sparano JA, Paik S (2008) Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 26(5):721–728
Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, Watson D, Geyer CE, Wickerham DL, Wolmark N (2010) Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 28(10):1677–1683
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American Society of Clinical Oncology/College of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH et al (2016) Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Nat Compr Cancer Netw 14(3):324–354
Ricks-Santi LJ, McDonald JT (2017) Low utility of oncotype DX® in the clinic. Cancer Med 6(3):501–507
Cress RD, Chen YS, Morris CR, Chew H, Kizer KW (2016) Underutilization of gene expression profiling for early-stage breast cancer in California. Cancer Causes Control 27(6):721–727
Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D (2005) Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 294(14):1765–1772
Rauscher GH, Silva A, Pauls H, Frasor J, Bonini MG, Hoskins K (2017) Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast Cancer Res Treat 163(2):321–330
Wheeler SB, Reeder-Hayes KE, Carey LA (2013) Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist 18(9):986–993
Rauscher GH, Silva A, Pauls H, Frasor J, Bonini MG, Hoskins K (2017) Racial disparity in survival from estrogen and progesterone receptor-positive breast cancer: implications for reducing breast cancer mortality disparities. Breast Cancer Res Treat 163(2):321–330
Daly B, Olopade OI (2015) A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 65(3):221–238
Acknowledgements
This research was supported by a grant from Susan G. Komen® GTDR16376189.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Press, D.J., Ibraheem, A., Dolan, M. et al. Racial disparities in omission of oncotype DX but no racial disparities in chemotherapy receipt following completed oncotype DX test results. Breast Cancer Res Treat 168, 207–220 (2018). https://doi.org/10.1007/s10549-017-4587-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4587-8